Section 3
This section is
dedicated to studies carried out on the DC-arcjet system. Chapter 5 describes the reactor design,
growth characteristics, torch-head flow properties and the experimental set-up
used in the various studies. The
results of a growth study, performed as a function of process conditions, are
also included in this chapter. Chapter
6 describes the results gained from studies concentrating on the addition of
nitrogen into the DC-arcjet reactor in terms of the characteristics of the deposited
films and the in-situ optical emission of the plume. The final chapter outlines a preliminary
Cavity ring-down spectroscopic study of the plume, with the detection of
excited state C2 molecules.
Chapter 5 :
Experimental set-up, data analysis and growth characteristics of the DC-arcjet
system
The aim of this
chapter is to describe the specifics of the DC-arc plasma jet system used
during the series of experiments carried out at Bristol. Section 1.6.4 described in detail the
fundamental and general aspects of the diamond deposition system, together with
an outline of previously published studies.
As the deposition performance of a reactor will be subject to its
design, it is essential to define its characteristics accurately.
Firstly, this chapter
deals with the reactor design and set-up.
Early growth studies, which provided information regarding the
optimisation of the deposition parameters, are also included in this chapter. The technical considerations for the
collection of optical emission and the detection of species via cavity
ring-down spectroscopy are described later.
5.1 Reactor design
The main components of
a general DC-arcjet CVD deposition system, previously illustrated schematically
in figure 1.15, will be discussed here in turn. The most important components are the torch-heads; these are
described first, followed by a account of the reactor set-up.
5.1.1 Torch-Head Arrangement
The torch-head
design used was provided and installed by the Aeroplasma Co. Designed around a concentric cylinder
arrangement, the central anode and cathode ensures an arc may be struck in the
gas stream, thus forming the plasma.
While many groups use a single torch head, the twin torch head
arrangement has been shown to improve the ignition and stability of a plasma
jet. The primary torch, from here on
termed the N-torch, has an outer metal shell that is negatively biased relative
to the secondary, P-torch. This design
feature is used to obtain an arc between the two torch heads and thus improves
ignition of the plasma jet. The twin
torch arrangement is shown schematically in figure 5.1.
![]()
![]()
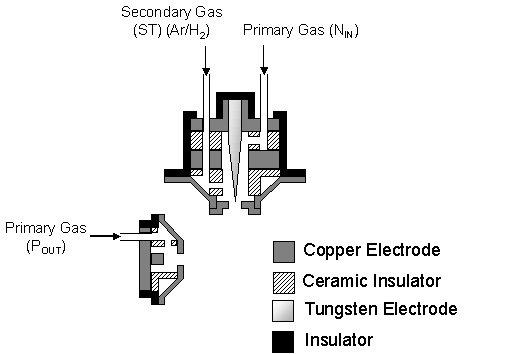
Figure 5.1 Schematic cross-section
of the Aeroplasma twin torch-head system.
Note the electric contacts and water-cooling supply and chambers have
been omitted for clarity. This figure
is not to scale, and is shown here only to illustrate the separate gas flows
through the two torch heads and the layout of the electrodes.
The torch-head
electrodes are composed of so-called ‘pure’ copper, with a purity greater than
99.9%, with the central N-torch cathode being made of tungsten. In order to prevent melting of the copper
electrodes a complex water-cooling system exists within the torch heads. The copper electrodes contain a system of
inner channels allowing the flow of high-pressure deionised water. However, studies have shown that while
water-cooling may prevent damage to the torch heads the large flow of gas
removes the majority of heat from the electrodes[1].
Electric power coupled
into the torch-heads, via feedthroughs, operates in a current led form with
variable voltage to control the total discharge power. The system has a maximum power discharge
rating of 10.0 kW (100 V, 100 A) with typical deposition conditions drawing
power at between 5.5 and
9.5 kW.
The flow of gases
through the torch heads is designed to propagate a stable, highly dissociated
plasma jet, as outlined in figure 5.2.
The gas flowing through the torch-heads are separated into primary and
secondary flows.
The primary gas flow
is solely argon (BOC, 99.95% purity).
Typical flow rates are between 7.5-10 standard litres per min (SLM)
through the N-torch and at a lower rate (0.5-0.75 SLM) through the P
torch. In this system the primary gas
flow is maintained at an input pressure of 4 Bar, throughout the deposition
process. These flows are normally
termed the NIN and POUT, from the incidence of the flow through the respective
torch-heads. These flows constitute the
majority of the total gas flow, with a main role as a carrier gas.
The use of large flow
rates of relatively inexpensive argon as a carrier gas is a common practice in
plasma jets. Argon, having an
ionisation energy of ~15.75 eV, is readily ionised within the torch-heads,
yielding free electrons in the plume.
While no studies have concentrated on the effect of argon on the diamond
deposition process, it is widely thought that argon plays a significant role in
gas-phase reactions as a third body1.
Ignition of the system
involves striking an arc within the torch-heads through which primary
argon flows, then striking an arc between the torch-heads an extended
plume is formed by coalescing of the two plasmas produced within the torch
heads. The high velocity argon plasma
jet consists of a stream of high temperature neutral and ionic argon species,
as well as free electrons.
The secondary gas flow
consists of a mixed flow of Argon and Hydrogen (BOC grade, 99.95% purity) at
flow rates of, respectively, 1.4 SLM and between 1.2 and 2.0 SLM, to a maximum
total flow of 3.5 SLM. The separate
flows are metered through MKS mass-flow controllers (MFC’s) before being mixed
in the gas line prior to injection into the N-torch head. The secondary flow input gas pressure is
typically maintained at 3.0 Bar.
Pressure differences between the primary and secondary gas flows serve
to protect the N-torch, as the secondary flow is injected downstream of the
primary, thus preventing backflow of gas.
The Aeroplasma torch-heads are designed to use a spiral induction flow
system that helps to stabilise the plume, lower electrode erosion and mobilise
the arc connection. The secondary flow
passes into the N-torch through a channel in a ceramic spacer. Laser machined holes, cut into the ceramic
at an angle incident to the plasma flow, are such that the gas injected into
the plume forms a spiral flow.
The gas flow and
electric discharge causes rapid thermal expansion of the gas which, when
channelled through the 2 mm diameter converging-diverging nozzle aperture
(N-torch), forms an expanded Ar/H2 plume.
The temperature of the plasma is sufficiently high to partially
dissociate the gas to form argon and hydrogen neutral and ionised species. The
gas supply to the DC-arc torch-heads is shown in figure 5.2.
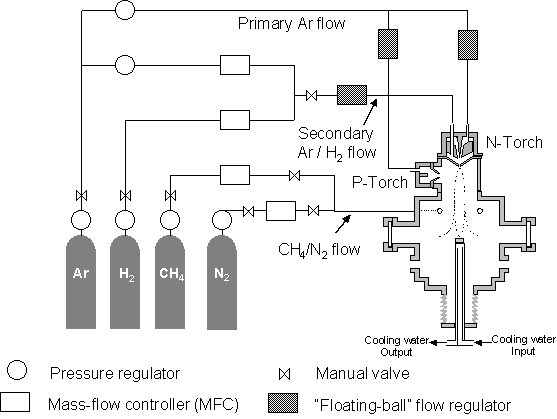
Figure
5.2 Gas flow line diagram
Hydrocarbon, methane
in the case of studies described in this thesis, is introduced into the chamber
via an annular injection ring. This
ring, situated 100 mm downstream from the N-torch nozzle exit, injects methane
metered via a MFC (MKS), directly into the chamber via a number of uniformly
distributed holes. It has been shown
that remote hydrocarbon injection increases the stability of the plume and
prevents the growth of amorphous carbon deposits on the nozzle exit[2]. Experimental[3]
and modelling[4]
studies have also suggested that the position of the injection point relative
to both the nozzle and substrate may be optimised to increase growth rate and
quality.
5.1.2 Reactor considerations
Downstream of the
nozzle, the plume impinges onto a water-cooled molybdenum substrate. Molybdenum is the most commonly used
substrate material in DC-arcjet CVD due to its high melting point, thermal
conductivity and ability to form a carbide layer. However, a number of groups have demonstrated growth onto other
materials e.g. Tungsten and Silicon.
The use of silicon has previously been explored and was found not to
withstand the thermal shock on ignition of the plasma jet.
Growth onto molybdenum
yields films freestanding in nature, i.e. no substrate material is
attached. This has significant
advantages over growth onto silicon wafers.
It has been considered that DC-arcjet deposited films may be of use in
the production of heat sinks, in the thermal management of high power diode
lasers and microchips, together with other applications, requiring the use of
thick-film diamond layers without a substrate material. Prior to deposition the molybdenum substrate
is polished using 1 mm grade diamond dust.
The pressure in the
reaction chamber is continually monitored and controlled via a MKS pressure
control system linked to an in-line butterfly valve connected, via vacuum
tubing, to an Edwards (E2M40) two-stage rotary vacuum pump.
The pressure is
measured continually, on the vacuum line from the reaction chamber to the
rotary pump, using a Pirani and a Baratron gauge. Prior to a deposition run the reaction chamber is evacuated to
<50 mtorr. While not evacuating the
chamber to a high vacuum, it is considered that the high gas flow argon purge,
used prior to ignition, efficiently removes the majority of residual gas-phase
impurities (i.e. from residual air).
Figure 5.3 shows the
apparatus and reactor service connections used in the deposition of films, with
particular reference to the vacuum apparatus.
Note that the symmetric vacuum tubing about the chamber ensures the
plume is stabilised about its centre.
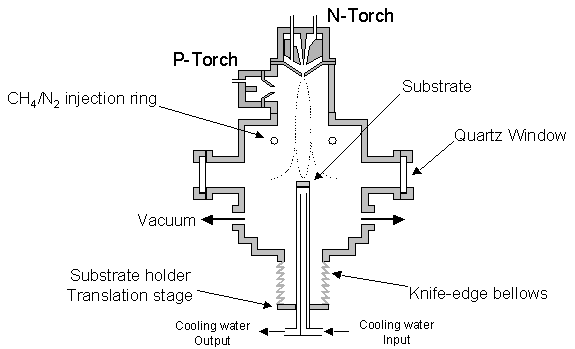
Figure 5.3 Schematic of reaction
chamber with particular reference to vacuum apparatus. Note that this diagram is not to scale and
for reasons of clarity all chamber water-cooling passages have been omitted.
Due to the high
enthalpy plume, removal of excess heat from the reactor walls, torch-heads and
substrate is a priority. Chilled
deionised water is supplied under pressure (40 psi) by a dedicated water
chiller and pump system (F&R RCU7).
Prior to being used as a coolant the water is passed through two 0.5 mm filters and a water deionising cylinder. Given the need to remove large quantities of
excess heat, and the fine bore channels that exist within the torch-heads, it
is prudent to provide cooling water at an elevated pressure. This is achieved via the use of a
high-pressure water pumping system (Fenner) which, under deposition conditions,
typically operates at
9 Bar.
The reaction chamber
is a hollow-wall stainless steel design allowing the flow of high-pressure
chilled water, thereby cooling the reactor walls. The reactor is equipped with side-ports, to allow viewing and
optical diagnostics of the plume, and are fitted with quartz windows. Although these viewing arms are not cooled,
measurements taken outside the chamber show the temperature of the arms to
remain below 45°C during
normal deposition conditions. The
viewing arms are positioned in a way to either allow viewing of the substrate
or to facilitate gas-phase diagnostics by providing a unhindered line of sight
through the reactor, close to the substrate surface.
The temperature of the
substrate is measured via an optical pyrometer (Agema two-colour) viewing
through a quartz window and focused onto the substrate. The non-greyness factor (a correction factor
used to allow for differences in the emissivity of a material at short and long
wavelengths) of the molybdenum substrate, used in the pyrometer calibration,
was set to be 0.8 throughout the studies.
Water-cooling is used
to maintain the temperature of the substrate.
The molybdenum substrate is positioned on the end of a stainless steel
conduit, providing both necessary cooling water and holding the substrate in a
fixed position. During the time span of
the studies describe within this thesis two designs of substrate and substrate
cooler were used, see figure 5.4.
During studies
focusing on nitrogen addition into the diamond depositing Ar/H2/CH4 gas mixture
and the OES of the plume (Chapter 6) the substrate cooler design A, outlined in
figure 5.4 was used. The substrate
diameter used with this design was 12 mm, with silver-DAG being used to ensure
high thermal contact between the copper mount and the back of the substrate.
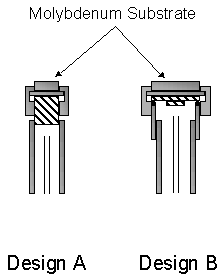
Figure 5.4 Cross-sectional schematic
diagrams showing the two designs of substrate cooler used during the studies
described in this thesis. Note this
diagram is not to scale.
Design B, used
throughout the CRDS studies (outlined in Chapter 7), allows a substrate of
diameter 26 mm to be mounted and efficiently cooled. In order to allow the substrate to reach temperatures conducive
to high-quality diamond growth a copper spacer is used. The copper spacer was machined in a ‘top
hat’ form to cool the Viton O-ring sufficiently.
Regardless of the
substrate cooler design used, the cylindrical cooling pipe is aligned in the
chamber through the use of a 3D-translation stage. The translation stage allows the substrate to be manoeuvred in
three dimensions relative to the incident plume. Knife-edge bellows (being air-cooled) allow the chamber pressure
to be maintained, while permitting substrate movement.
The total substrate
cooling-water flow was maintained at 1 l/min throughout the studies. Any change in the cooling-water flow to the
substrate was shown to have a minimal effect on the substrate temperature,
although by using a compressed air/water spray system Breiter et al.[5]
have shown improved control of the substrate temperature. The water-cooling system allows the
distribution of water between the torch-heads, substrate, reactor walls and a
copper flange onto which the torch-head arrangement is mounted. During typical deposition conditions the
flow rates are distributed thus:
torch-heads 10.5 l/min, substrate 1.0 l/min, reactor walls 2.0 l/min and
copper flange 3.0 l/min.
5.2
Growth
Studies
The large number of variable factors (e.g. gas flows, discharge power,
chamber pressure, substrate temperature, torch nozzle-substrate separation etc.)
makes the DC-plasma jet system very flexible.
Studies were undertaken to optimise the process conditions with respect
to deposition rate, growth quality and facet formation.
Films were deposited and analysed using Scanning Electron Microscopy (SEM)
and Laser Raman Spectroscopy (LRS), described in appendix 7. SEM analysis reveals both the facet
formation and crystallite sizes of the growing surface and, by cleaving the
grown films and examining the cross section, the growth rate. LRS allows a measure of the diamond (sp3)
and graphitic (sp2) carbon content of the film to be obtained.
5.2.1 Influence of methane
addition
Studies have previously shown that increasing the hydrocarbon content in
the gas-phase increases the growth rate, but may also have a detrimental effect
on the film quality[6]. A series of films were deposited for one
hour with the process conditions specified below and various methane additions.
Ar flow rate NIN
= 7.5 SLM
POUT = 0.75 SLM
Secondary Ar flow = 1.4 SLM
H2 flow rate = 1.25 SLM Discharge
power » 5.50 kW
Chamber pressure = 200 Torr Substrate
temperature = 820°C
Deposition duration = 1 Hour
Cross-sectional SEM images yield the growth rate and show the nucleation
and coalescent nature of the crystal growth.
Figures 5.5 show two such SEM images for the addition of (i) 0.72% and
(ii) 1.44% methane respectively.

Figure 5.5 Representative SEM
images showing the change in growth rate and crystallinity obtained by changing
the methane addition with a common growth scheme.
Both SEM images show high nucleation density at the substrate, columnar
growth and the production of large crystals as growth proceeds. The deposition rate is shown to increase
with increasing methane addition from ~110 to 167 mm hr-1 for methane additions of 0.72 and 1.44 %
respectively. It is also worth noting
that the film deposited with a higher methane percentage is considerably more
uniform in terms of thickness throughout the diameter of the film.
Figure 5.6 illustrates the general trend in growth rate as a function of
additional methane (quoted as the percentage of total gas flow).
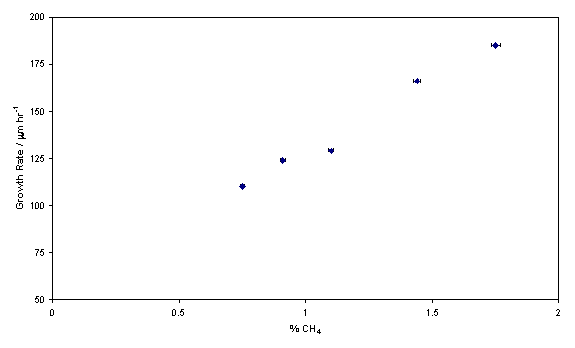 Figure 5.6 Trend showing growth rate, obtained from
cross-sectional SEM analysis, as a function of additional methane volume
fraction. The x-axis error is
set as 1 %, the standard error quoted by the MFC manufacturer.
Figure 5.6 Trend showing growth rate, obtained from
cross-sectional SEM analysis, as a function of additional methane volume
fraction. The x-axis error is
set as 1 %, the standard error quoted by the MFC manufacturer.
Clearly methane addition directly influences the deposition rate, the
quality of the film also depends on the relative quantity of hydrocarbon
added. LRS has been used to show how
the methane addition affects the film quality in terms of the diamond-graphitic
carbon content. Figure 5.7 shows two
representative Raman spectra. As
discussed in appendix 7, LRS can be used to reveal information about the ratio
of the sp3 (Raman peak centred at a Stokes’ shift of 1332 cm-1) and sp2 (broad
feature centred about 1550 cm-1) carbon content in the grown film.
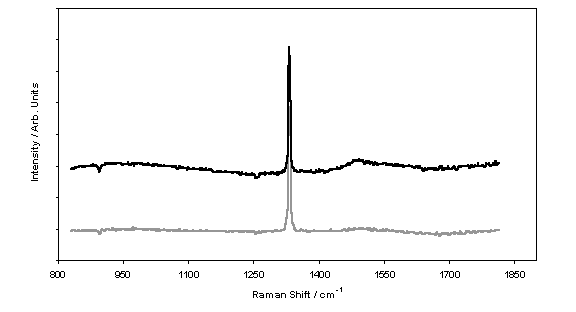
Figure 5.7 Background-corrected
Raman spectra illustrating the change in film quality with methane
addition. The vertically-shifted upper
trace corresponds to a film deposited with 1.15 % methane, while the lower
trace relates to a 0.75 % addition.
Both spectra were taken at the centre of each film using 325 nm laser
excitation.
5.2.2 Influence of substrate temperature
Studies have
previously identified the temperature regime in which diamond deposition is
optimised in terms of quality and growth rate6. Figure 5.8 shows the surface topography of three films deposited
at different substrate temperatures, but otherwise common process conditions of
0.75% CH4, chamber pressure of 200 Torr, discharge power of ~5.50 kW and deposition duration of 1 hour. The crystallite size is different at the
altered substrate temperatures and serves to highlight that both the gas-phase
and surface chemistries are important in diamond growth. The SEM images show a change in the
crystallinity with changing substrate temperature, however it is very difficult
to characterise the crystal facets present.
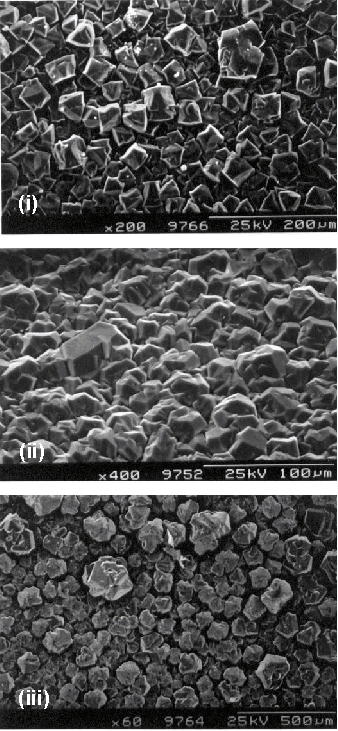
Figure 5.8 SEM images showing the
effect of substrate temperature on the surface crystallinity. Images correspond to substrate temperatures
of (i) 740, (ii) 820 and (iii) 900°C respectively. Note the change in scale between figures and
that while images (i) and (iii) are plan views, image (ii) was taken at ~45° to the surface
normal.
5.2.3 Chamber Pressure
The pressure in the
chamber influences the plume stability, the size of the visible region of the
plasma, the absolute concentrations of species within the plume (and their
spatial variation) and thus the growth characteristics.
Increases in chamber
pressure cause an increase in the number of species collisions in the gas-phase
environment, which may lead to increased levels of growth species. However, in a DC-arcjet reactor there is an
inevitable trade-off between the stability and spatial uniformity of the plume
(significantly improved at low chamber pressure) and the gas-phase species
density.
Figure 5.9 illustrates
this point with SEM images showing two films deposited at chamber pressures of
(i) 50 and (ii) 200 Torr, with all other process conditions maintained as
identical as possible. These films were
deposited using the common process conditions; 0.75% CH4, substrate temperature
of 820 °C, discharge
power of ~5.50 kW and
deposition duration of 1 hour. Note
that although at higher chamber pressures the growth rate is increased, the
film is less uniform, both on a local scale and radially across the entire
substrate.
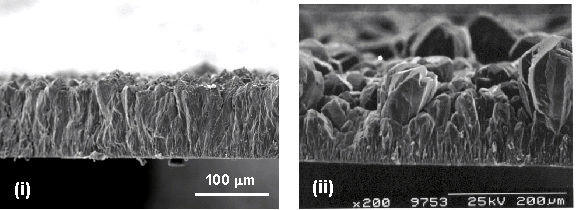
Figure 5.9 Representative SEM
images showing the alteration in growth rate and surface cystallinity with
changes in chamber pressure. Note the
scale bars differ between images.
5.2.4 Discharge Power
Altering the discharge
power operating through the torch heads influences the average plasma
temperature and thus the degree of species dissociation. Clearly the degree of dissociation of
species within the plume will dictate the quantity of both the growth species
and atomic hydrogen, at the growing surface.
However, studying the effect of discharge power on film growth
characteristics is hampered by the substantial change in substrate temperature
as a function of power change.
5.3 Experimental Set-up for OES
collection
This section sets out
to describe the experimental set-up and considerations required to undertake
the spatially resolved optical emission studies documented in chapter 6. These were specifically directed at
explaining the effects of nitrogen addition to a typical Ar/H2/CH4 gas mixture.
The reaction chamber
is unaltered from the description given earlier in this chapter. Figure 5.10 provides a schematic
illustration of the experimental apparatus used for film deposition and for
collection of optical emission from the plume.
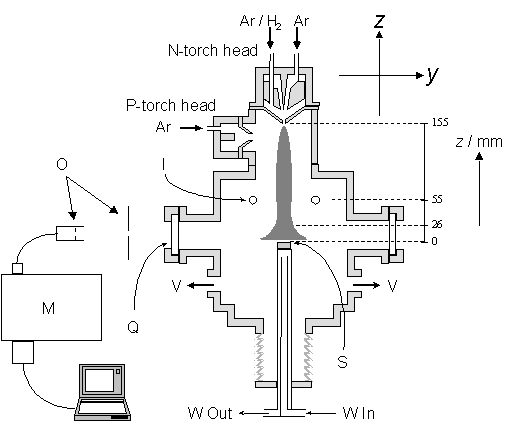
Figure 5.10 Schematic
representation of the apparatus required to deposit films and collect spatially
resolved optical emission. Key to
figure: I, injection ring for CH4 (and N2); V, connections to rotary pump; W,
substrate cooling water (input and output); all other items are detailed in the
text.
The plasma jet
propagates in the –z direction, with the surface of the substrate (S)
positioned at, and defining, z = 0.
Optical emission was collected in the –y direction, viewed
through a quartz window (Q), defined by a train of iris diaphragms and a fibre
optic bundle (O), dispersed with the monochromator (M) and detected with the
CCD array. Note that in Figure 5.10 the
x-axis is orthogonal to the plane of the figure. The plasma impinges normal to the surface of
the Molybdenum substrate, which, in the OES experiments reported in chapter 6,
is fixed at a position 155 mm from the nozzle exit of the N-torch.
All films deposited
during this study of the effects of nitrogen addition were grown at a substrate
temperature of 880°C and a
chamber pressure of
50 Torr. Each deposition lasted one hour, with
identical CH4/H2/Ar flow rates, variable N2 additions, and a constant input
power of 5.9 kW (78 V, 76 A). The
resultant films delaminate on cooling and were analysed as free-standing. Growth rate and surface topology were revealed
by SEM (Oxford Instruments), laser Raman spectroscopy (Renishaw, He-Cd laser
excitation at 325 nm) provided an indication of film quality, and the degree of
nitrogen incorporation in the films was revealed via SIMS.
This study allowed the
collection of spatially resolved distributions of optical emission from
electronically excited H atoms, and C2 and CN radicals. A
2 mm diameter column
of the plume emission was defined by a combination of two irises positioned, in
series, in front of a quartz fibre optic bundle all of which were mounted on a
common two-dimensional translation stage.
Optical emission was collected at 1 mm intervals, at a fixed x-position, along the
z-axis, in the region 0 < z
< 26 mm, where z = 0 mm is defined by the substrate surface and
determined by laser alignment.
Collection was also carried out in the x-axis in the region, –4 < x
< 16 mm, for a number of different fixed z
values, each separated by 5 mm, in front of the substrate surface and
perpendicular to the plasma flow.
Spectra were collected
using a UV extended charge-coupled device (CCD) array detector, mounted onto
either a 12.5 cm monochromater (Oriel Instaspec IV, 600 lines mm-1 ruled
grating) or a 0.5 m monochromator (Spex 1870, 2400 lines mm-1 holographic
grating) depending on the wavelength range and resolution required. The CCD array allows simultaneous collection
of all emission within the wavelength range of interest, with collection and
processing carried out on a connected personal computer. Frequency calibration of the Oriel
monochromator was carried out by comparison with the known mercury transitions
from the fluorescent tube room lights.
The Oriel
monochromator allows the simultaneous collection of a 300 nm wavelength
range. This range extends to envelope
transitions from the Swan band system of C2, H (a, b, and g Balmer transitions) and a CN system, all visible in
emission from the plume. By using a
previously determined scaling factor[7],
the wavelength dependent intensities may be corrected for the response function
of the monochromator and for the frequency dependence of the quantum efficiency
of the CCD array.
5.4 Experimental set-up required for CRDS
study
This section describes
the modifications made to the DC-arcjet reactor, additional apparatus and the
experimental outline for the CRDS study of C2.
The CRDS studies
required modification of the chamber to allow the definition of an optical
cavity through its length, perpendicular to the direction of plasma flow (along
axis y in figure 5.11). Figure
5.11 schematically outlines the apparatus used in this study. The addition of extension arms to the
chamber was deemed necessary to protect the CRDS mirrors from highly reactive
gases, which may degrade the reflectivity.
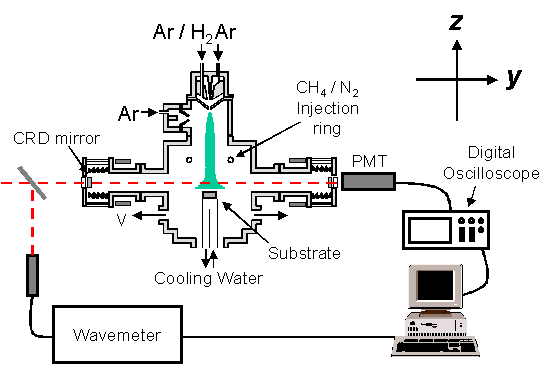
Figure 5.11 Schematic
representation of the apparatus for CRDS study.
Laser radiation was
generated from a Spectra-Physics PDL-3 tuneable dye laser, pumped by a
Spectra-Physics DCR-2A Nd-YAG, operating at a pulse rate of 10 Hz. Output radiation from the dye laser has a
specified bandwidth of 0.05 cm-1 in the visible. Frequency scans over the required range were facilitated with an
in-house made driver unit which steps the angle of the diffraction grating in
the dye laser. Light generated at ~515
nm was directed into the chamber via a series of prisms and optics to allow
versatile adjustment of the beam height and angle into the cavity while
minimising any divergence or convergence.
The cavity is defined
by two highly reflective mirrors (99.93% reflective at
515 nm) separated by a
fixed distance of 101 cm. The mirrors
are held in stainless steel mounts which are fixed onto the main chamber via
flexible knife-edge bellows.
Micrometers, fixed between the mounts and the chamber, allow the mirrors
to be adjusted and fixed so as to maximise reflected light within the cavity. The fraction of light that passes through
the exit mirror was detected with a photomultiplier tube (PMT) with connection
to a digital oscilloscope (LeCroy 9361).
Signal collection by the oscilloscope was triggered by the Q-switch
output from the Nd-YAG laser.
The PMT and exit
mirror were shielded from external light by using a cover, which demonstrated a
reduction of the noise on the observed signal.
The frequency of the laser light was constantly monitored with a
wavemeter (Coherent Wavemaster) operating in pulse collection mode and set for
self-calibration prior to each scan.
Signals from both the wavemeter and the oscilloscope were sent to a PC
for collection, processing and storage.
The use of the in-house written data collection program Drive, allows
simultaneous collection of the laser wavelength and the ring-down time. The program also converts the ring-down time
to that of a decay co-efficient, which, as described in chapter 7, may then be
used to obtain absolute measurements of absorption.
CRDS, being an
absorption based technique, is not highly subject to changes in the pulse
energy and therefore does not warrant a constant laser power reading. In order to correct for the changes in laser
power as a function of wavelength, a background scan is subtracted from the
normal scan. The collection of the
background scan was carried out by operating on an Ar/H2 plasma and thus only
providing information of the ring-down dependence with wavelength.
References