Chapter 6 : Nitrogen addition to a DC-arcjet reactor
This chapter
serves to present and analyse the results of both spatially resolved optical
emission and film deposition studies, carried out in the DC-arcjet reactor,
with particular reference to the addition of trace quantities of nitrogen.
The addition
of trace quantities of nitrogen into the feed gas of hot filament[1],
microwave[2]
and oxy-acetylene torch[3]
CVD systems during diamond deposition has been shown to enhance both the growth
rate and deposition quality. In all
systems a peak in both the film quality and growth rate is obtained with the
introduction of controlled quantities of nitrogen. The work carried out, and documented, in this chapter aims to
study the effect of additional nitrogen on both the gas-phase environment, via
Optical emission spectroscopy (OES), and the characteristics of the grown
films. The experimental specifics have been described
previously in chapter 5.
6.1
OES Measurements
The use of OES in the characterisation of a gas-phase
environment is commonplace in the field of plasma diagnostics due to the ease
of data collection and non-invasive nature of the technique. However, interpretation of the emission data
is complicated by a number of factors.
OES only gives information about excited state species which, due to
their low relative abundance in the diamond-depositing gas-phase environment,
are generally considered to be unimportant in the diamond deposition
process. Relating the excited state
information to that of the more abundant and functional ground state species
requires detailed knowledge of the production and collisional quenching
mechanisms.
While the interpretation of OES suffers from a number
of complications, its use as a plasma diagnostic is justified in the study of
species as a function of spatial variation and changes in process conditions.
OES
measurements taken in this study all used a fixed Ar/H2/CH4
feed gas ratio and flow rate (Ar 87.83%, H2 11.59%, CH4
0.58%, total flow rate
13.8 slm) with
the addition of known quantities of N2 in the range 0-100 sccm.
Figure 6.1
shows typical wavelength-dispersed optical emission spectra of both an Ar/H2/CH4
(upper trace) and Ar/H2/CH4/N2 (lower trace)
plasmas in the DC-arcjet environment.
Clearly the emission spectra of both plasmas are dominated by the C2
(d3Pg®a3Pu) Swan band
system but, as figure 6.1 shows, emission from atomic hydrogen (Balmer-a transition,
henceforth Ha) at 656.3 nm
is also visible.
The CH4/H2/Ar/N2
plasma spectra also includes emission from the CN(B2S+®X2S+) system
(hereafter also referred to as CN (B®X)) at ~388 nm. CH
(A2D®X2P) emission at
~431.4 nm is discernible, but so weak in comparison with the Swan band system
that it is not considered in this study.
All emission lines from Ar (neutral and ionic) observed from the pure Ar
plasma are quenched on addition of H2, thus preventing their use in
actinometric measurements.
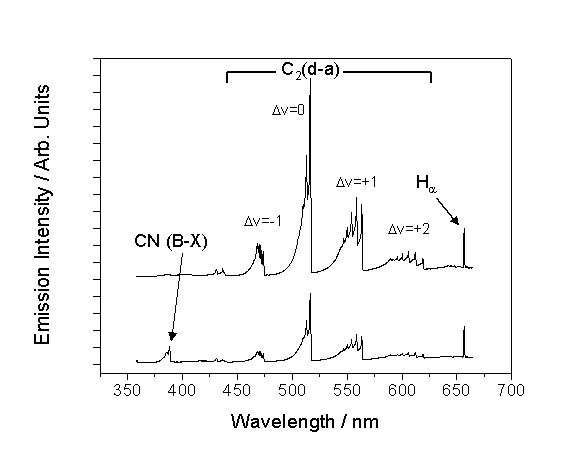
Figure
6.1 Wavelength-dispersed optical
emission spectra of
an Ar/H2/CH4 plasma (upper trace) and with the addition of
10 sccm nitrogen (lower trace). Both spectra were measured at
z = 10 mm and x = 0 mm
with a 2 mm diameter viewing column and the Oriel Instaspec
IV monochromator. Chamber pressure was maintained
at 50 Torr.
Throughout the
OES studies the measurements of the C2 and CN emission intensities,
taken as a function of process condition, were obtained at a fixed wavelength
and not as the integral of the vibrational band. This method was chosen as the overlapping of visible emission
from a number of species and vibrational systems complicates the spectrum. For example, within the region enveloped by
the C2 (d®a)(Dv=0) band,
emission from Hb also appears
at ~486.1 nm. Prior measurements[4]
show that the emission intensity obtained from the integral of a vibrational
band and from a fixed wavelength both follow the same trend with changes in the
process conditions.
For data used
in this study the following wavelengths were probed. For C2 emission, the (0,0) bandhead at 516.5 nm was
monitored. In the case of the CN(B®X) the
emission at 388.8 nm was collected, while Ha was monitored at 656.2 nm. Since the aim of this study was only to show
species intensity trends in terms of either spatial distribution or process
conditions, no attempts were made to establish absolute emission intensities.
Figure 6.2
shows the measured variation of the CN(B®X), Ha and C2(d®a) emission
intensities as a function of added N2. The growth in CN(B®X) emission clearly implicates nitrogen as a species that
participates in the gas-phase chemistry within the plasma plume, while the
plots of C2(d®a) and Ha emission
intensities serve to illustrate the substantial quenching induced by the
addition of just a trace of N2.
Consistent with the previous discussion regarding the inevitable presence
of some background N2 in the process gas mixture, very weak CN(B®X) emission is
observed with (nominally) 0 sccm added N2; extrapolating the data
displayed in fig. 6.2 provides an upper limit estimate of 2.2 sccm (~160 ppm)
as the 'flow rate equivalent' N2 content under these conditions.
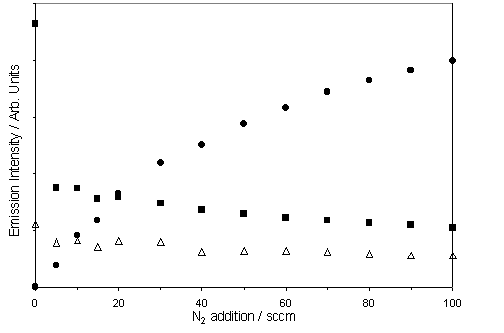
Figure 6.2 Variation in OES intensity of CN(B®X) (=), Ha (r) and C2(d®a)(0,0) (<) from intensity
measurements taken at 388.83, 656.28 and 516.52 nm respectively (frequency
calibration determined from mercury transitions in fluorescent lighting).
Previous
measurements of the C2(d®a) Dv
= 0 progression, recorded at higher resolution, using the Spex 1870
monochromator, showed no obvious variation in band contour either with process
conditions or with spatial location, though it is worth noting that this did
not include the ~1 mm closest to the substrate surface (i.e. the region containing the boundary layer). This encourages the assumption that spatial
variations in emission intensities, monitored via the intense (0,0) band head
at ~515 nm, are representative of the entire distribution of emitting C2
species.
6.1.1 The C2 molecule
The study of
the C2 molecule, in particular the Swan band system, is of
particular interest in the field of astrophysics where emission spectra from
comets and the absorption spectra of stellar atmospheres have been studied.
At this point
it seems prudent to mention some details regarding the electronic configuration
of the C2 molecule. If we
consider the electronic configuration of the homonuclear diatomic molecule,
from the Aufbau principle, ground-state C2 has the electronic
configuration,
![]()
![]()
With
multi-electron molecules it is feasible that electron promotion to the lowest
energy excited-orbital may result in an unexpected low-lying excited electronic
state, due to the presence of electron-electron repulsion. This is the case with C2, where
excitation of an electron to a higher orbital, results in a low-lying excited
energy state with the electronic configuration,
![]()
![]()
This low lying
excited state, labelled a3Pu, is only 716 cm-1 above the ground state and is
appreciably populated even at modest temperatures. This study concentrates on the Swan band system, C2(d3Pg®a3Pu), relying on
information gained from the emission between two excited states, and thus
reveals little information about the potentially more important ground state
species. The emitting d3Pg state is
thought to be produced in the DC-arcjet system via electron impact excitation
of the abundant lower lying a3Pu state[5]. Boltzmann calculations show that at a
typical plume temperature of 3000 K the number of C2 species in the
a3Pu state exceeds
that of C2 in the ![]() ground state.
ground state.
6.1.2 Spatially resolved OES measurements
Figures 6.3(a)
and (b) show plots of the spatially imaged C2(d®a) and CN(B®X) emission
intensities (at ~515 nm and ~388 nm, respectively, using a 2 mm diameter
viewing column) as a function of position along z, measured from the substrate surface.
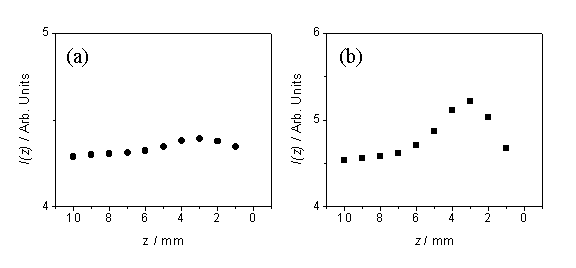 Figure 6.3 Spatially imaged emission intensities as a
function of z for (a) C2(d®a) and (b) CN(B®X) measured at x = 0
mm
Figure 6.3 Spatially imaged emission intensities as a
function of z for (a) C2(d®a) and (b) CN(B®X) measured at x = 0
mm
The local
maxima in emission intensity at a distance of ~3
mm from the substrate surface, for both species, mimics previous OES studies
carried out on DC-arcjet reactors[6]. Yamaguchi et al. have commented that it is probable that the maxima is not
due to increases in species density, but rather to an increase in the local gas
temperature, as the high velocity-high enthalpy species strike the substrate,
forming a shock wave close to the substrate surface.
As the plume
is cylindrical in nature any emission intensity measurements, taken as a
function of co-ordinate x, will be viewing a column of emission, the
thickness of which will depend on the x position. In order to ascertain the emission intensity
as a function of a radial co-ordinate, an Abel-inversion is performed on x
co-ordinate datasets.
6.1.3 The Abel Transform
The Abel transform is a mathematical function that allows the derivation of a radial dependence to be obtained from taking parallel chords through a cylindrical or spherical object. Its use is prevalent in plasma diagnostics in such systems that allow a number of assumptions to be made. These assumptions relate to the axial symmetry of the plasma and the degree of optical transparency. Both are essential to the analysis, which requires information on the emission throughout the extent of the line-of-sight. Figure 6.4 outlines the variables associated with the interpretation of an Abel transform.
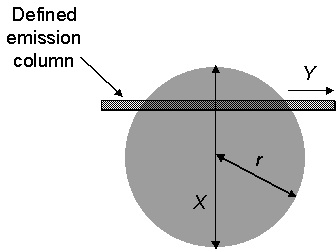
Figure 6.4
Outline of variables required for Abel transform of cylindrical plasma.
Application of
an Abel transform to the line-of-sight data set, enables derivation of the
radial dependence of emitting species within the plasma[7],
if we assume that the plasma is optically thin at the emission wavelengths of
interest. However, such remains to be
proven for the measurements reported here, particularly in the case of CN where
the measured emissions terminate on the ground state, but the trends deduced in
what follows remain valid even if the measurements are affected by preferential
self-absorption at x ~ 0 mm.
All measured
species specific lateral emission intensity distributions, I(x), appear
symmetric about x = 0 mm (as in
figure 6.5), thus satisfying the requirement of cylindrical symmetry for Abel
inversion. Knowing I(x) throughout the
range x = 0 to x = R (here chosen as 20
mm), Abel inversion yields the radial distribution of emitting species, i(r), via the
integral:
![]() Equation
6.1
Equation
6.1
The i(r) profile so
deduced for the case of C2(d®a) emission measured at
z = 6 mm is shown in fig. 6.5.
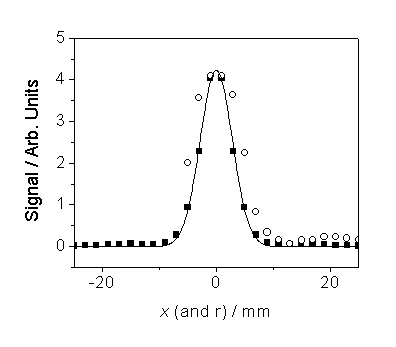
Figure 6.5
Abel-inverted C2(d®a)
emission intensity as a function of radial co-ordinate, r (n),
together with a data-smoothing function.
The raw C2(d®a)
emission data are also shown as a function of x (™).
As the raw
data in figure 6.5 show, the emission intensity points are collected at 2 mm
intervals. An Abel inversion function
is applied to the raw data thus yielding the Abel-inverted emission
intensities, which then have a data-smoothing function applied.
Given numerous
such profiles, taken at many z values
for the CN(B®X), C2(d®a) and Ha emissions, it
is possible to generate spatially resolved emission intensity maps for each of
these species. Emission was collected at 1 mm intervals along the z-axis in the region 0 < z < 26 mm (defined in figure 5.10)
and at 2 mm intervals in the range -4 < x
< 16 mm (i.e. out of plane depicted in figure 5.10) for several different
fixed z values, each separated by 5
mm, in front of the substrate surface and perpendicular to the plasma flow.
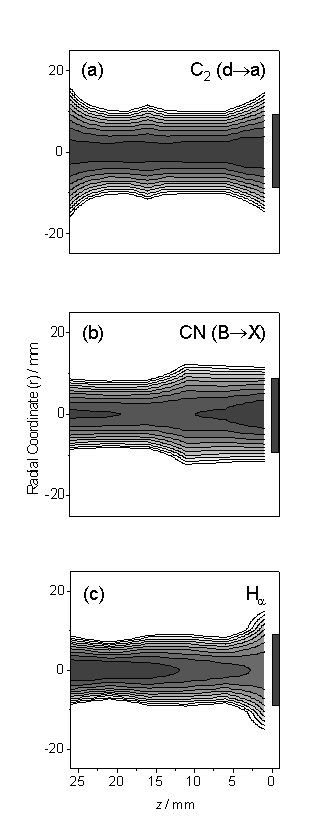
Figure 6.6 Spatially resolved
emission intensity maps for (a) C2(d®a), (b) CN(B®X) and (c) Ha. Note that each figure has an
independent logarithmic 10-point scale where high species intensity corresponds
to a darker grey.
Figs. 6.6
(a)-(c) show such plots, as a function of z
(horizontal axis, with the front face of the substrate at z = 0 indicated) and radial co-ordinate,
r.
All show some spatial inhomogeneity, with maximum emission intensities
at the plume centre (x ~ 0). The CN and C2 emission profiles
show similar full width half maxima (FWHM) values in r, indicating efficient mixing within the plume, but the radial
emission profile for Ha is narrower
and more localised in the upstream, hotter, region of the plasma jet.
Such trends
have been reported previously, and discussed, following analysis of plume
emissions from a lower power (2.3 kW) DC-arc plasma jet operating on an Ar/H2/CH4
gas mixture6.
All are
consistent with formation of C, N and H atoms by thermal decomposition of H2,
CH4 and N2 in the hottest regions of the plasma, and the
increasing importance of subsequent recombination reactions to form species
such as C2, CN, CH and H2 further downstream, at cooler
plasma temperatures. Where the plasma
impinges on the substrate surface the large kinetic energy associated with the
gas flow is converted into thermal energy, resulting in local gas heating.
Interpretation
of the results of OES studies requires some caution, given the nature of the
high velocity flux of species in the DC-arcjet environment. Estimates of the average number of
collisions that a species may undergo between exiting the arcjet nozzle and
striking the substrate based on Maxwell-Boltzmann statistics, have shown that
(assuming a pressure of 50 Torr and an approximate gas temperature of 3500 K)
the average number of collisions is of the order of 25.
As the
radiative lifetime of the d3P state of C2 is approximately 170 ns, many of the
species will have insufficient time to reach thermal equilibrium before
emitting. The interpretation of OES
data, in terms of relating excited-state emission intensity to ground-state
species number densities, requires the assumption of thermal equilibrium. While actinometry is commonly used to tackle
this problem all argon emission lines are quenched on the addition of hydrogen.
This study
confirms the production of CN, which in the high enthalpy plume may be produced
via a number of energetically favourable routes. Rudimentary studies of the gas-phase reaction scheme GRIMECH 3.1[8]
(see Appendix 6) identify nitrogen and nitrogen containing species as reactive
at temperatures reached within the plasma jet system. However, the H/C/N gas-phase chemistry scheme is highly complex
and strongly dependent on the gas temperature.
For this reason the gas-phase chemistry within the DC-arcjet reactor
needs to be studied in more detail, and while previous studies of H/C and H/C/N
chemistries at high temperatures have focused on computer simulation of the
reactor, further experimental studies are also required to support simulation
findings.
Finally, it is
worth noting that the present work supports previous suggestions6
that the observation of high C2 emission intensities in a DC plasma
jet correlates with higher quality diamond growth. This finding is in contrast to some of the earlier studies of
microwave plasma enhanced CVD which found strong C2 emission to be
an indicator of degraded diamond film quality[9].
6.2 Film
Deposition
The influence
of nitrogen addition was also studied in terms of the films deposited. While the growth rate and film quality are
of obvious importance, this deposition study was also interested in the
formation of diamond films with the incorporation of nitrogen. While natural diamond contains nitrogen as a
trace impurity, attempts to synthesize electronically useful nitrogen-doped
diamond by CVD techniques have, so far, proven disappointing.
For the
purposes of comparison a series of films were deposited with identical Ar/H2/CH4
feed gas ratio and flow rate (Ar 87.83%, H2 11.59%, CH4
0.58%, total flow rate 13.8 slm), with the addition of known quantities of N2
in the range 0-20 sccm. Each film was
deposited for one hour with a chamber pressure of 50 Torr and total input power
of 5.9 kW (78 V, 76 A). All films
delaminated on completion of the growth run and were analysed as free-standing
samples by a number of techniques. Each
surface diagnostic was undertaken on the centre of the circular samples.
6.2.1 SEM analysis
The use of
Scanning Electron Microscopy (SEM) reveals the growth rate, via cross-sectional
analysis, and also the surface topology.
Analysis was carried out on an Oxford Instruments SEM system with prior
gold coating of the sample surface. In
order to obtain both growth rate and an indication of the surface topology the
films were cleaved into sections and mounted at an approximately 45° angle to the
mount surface. This allows
visualisation of the cross section and the top surface while allowing a
sufficient working distance.
Figure 6.7
shows cross-sectional SEM images obtained as a function of additional
nitrogen. Note the scales are not
common between each image.
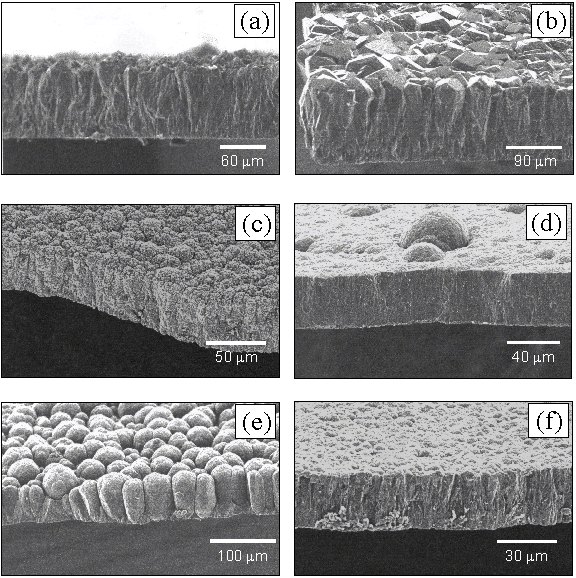
Figure 6.7
Cross-sectional SEM images of films grown with nitrogen addition of (a)
0, (b) 1, (c) 3, (d) 5, (e) 10 and (f) 20 sccm respectively.
From analysing
the SEM images it is clear that films grown without additional N2
are predominantly (111) faceted, and exhibit twinning and secondary nucleation
while, with the addition of 1 sccm N2, a central region of largely
(100) growth is formed, in which some of the crystallites exhibit facets
approaching ~60 mm in
size. Further addition of N2 promotes the growth of
ballas-type features.
Clearly N2
addition to the process gas mixture affects the film growth rate and
morphology. Figure 6.8 graphically
summarises the influence of nitrogen addition on the growth rate.
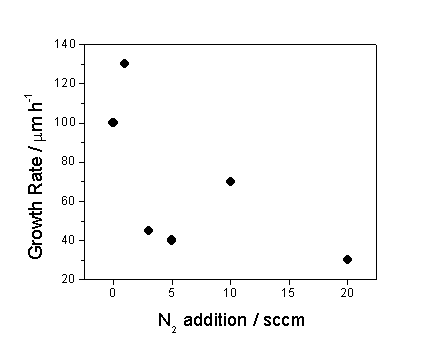
Figure 6.8
Plot showing the influence on growth rate from cross-sectional SEM
analysis as a function of nitrogen added during deposition.
Figure 6.8
shows the measured growth rate (mm hr-1) generally decreasing with increasing N2;
since the films grown at higher N2 partial pressures are
increasingly graphitic, and thus have lower density, the growth rate defined as
deposited mass per hour would fall off even more steeply with increasing N2.
6.2.2 Laser Raman analysis
Laser Raman
spectroscopy was used to assess the quality of the as-grown films using a
Renishaw system with excitation from a He-Cd laser operating at 325 nm. Representative background-corrected Raman
spectra of films grown with the addition of, respectively, 0 and 10 sccm of N2
to the process gas mixture are shown in figure 6.9.
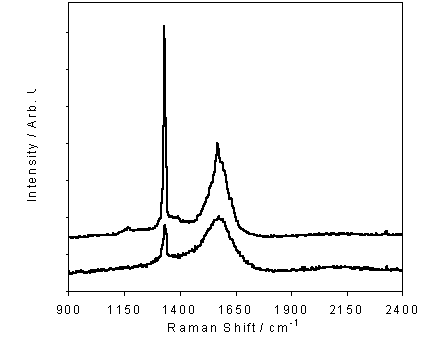
Figure 6.9
Representative Raman spectra of films deposited with the addition of 0
(upper trace) and 10 (lower trace) sccm nitrogen. Note that the spectra have been vertically shifted for
clarity. Both spectra have been
background corrected to allow for the underlying one-phonon transition.
Figure 6.9
clearly shows the Stokes shifts associated with sp3 carbon at ~1332
cm-1 and the broad sp2 carbon feature centred at ~1550 cm-1. While Raman cannot be used to reveal the
quality of growth on an absolute scale, it may be used to provide an indication
of the film quality by comparing the intensities of the sp3 and sp2
features. Figure 6.10 summarises the
findings of this study, where film quality here is represented by the quotient,
QD, for which:
QD = ID/(ID+IG), Equation 6.2
where ID and IG are, respectively, the relative intensities of the sp3
C peak (at a Stokes shift of ~1332 cm-1) and the sp2 C
feature (centred at ~1550 cm-1).
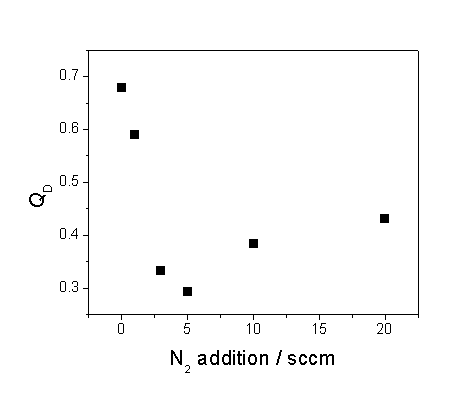
Figure 6.10 Plot showing the
influence on film quality, QD,
as a function of additional nitrogen.
This figure
illustrates the general reduction in film quality that accompanies increased N2
flow rates.
Studies in
other CVD environments have generally shown some improvement in diamond film
quality upon small (ppm) N2 additions, followed by a decline at
higher levels of added nitrogen. Such
an effect is consistent with the model of Moustakas et al.1,
which predicts that both n- and p- doping of diamond reverses the thermodynamic
stability between diamond and graphite favouring the growth of diamond over
graphite.
However, we
see only a reduction in QD with
increasing N2 additions, hinting at an obstructive role for nitrogen
in the diamond step growth mechanism.
This may reflect the poorer base vacuum of the large DC-arc plasma jet
reactor, such that it is always operating at a background partial pressure of
air and thus at nitrogen concentrations above those for optimal diamond film
quality. Consistent with this, we
observe a weak Raman feature at
~2328 cm-1
which, it has been suggested[10],
should be associated with a carbon-nitrogen stretching mode. This feature is present in all Raman spectra
measured in this study, including those of films grown in a standard CH4/H2/Ar
gas mixture with no intentionally added N2. Unfortunately, it is not sufficiently
intense for reliable inter-film comparisons.
6.2.3 Photoluminescence (PL) spectroscopy
Following on from the Raman study, PL spectra were obtained from a Renishaw system operating at 325 nm, with all measurements taken at room temperature. The use of PL is of particular interest in the analysis of nitrogen doped diamond films, as vacancies in the lattice formed by the inclusion of nitrogen are detectable. Figure 6.11 shows two representative PL spectra for films deposited with the addition of 0 (upper trace) and 20 sccm (lower trace) nitrogen.
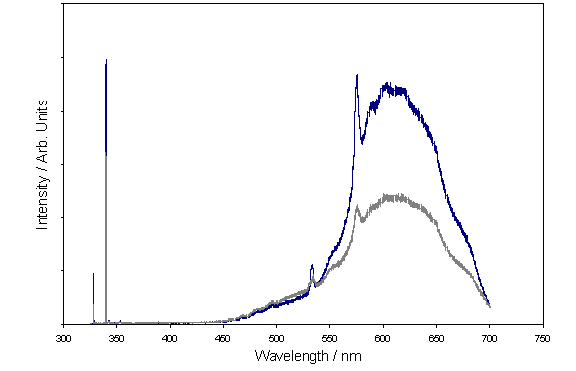
Figure 6.11
Representative PL spectra of films grown with the addition of 0 (upper
trace) and 20 sccm (lower trace) nitrogen.
From the PL
spectra the following assignments may be made.
The 575 nm (2.154 eV) peak corresponds to a nitrogen neutral [N-V]0
centre and is evident on all spectra.
Neutral [N-V] vibronic modes are all visible in the region 580-640
nm. The feature at 638 nm is commonly
associated with the negative
[N-V]-
centre and does not appear on the film deposited in the absence of nitrogen.
The fact that
nitrogen vacancies are detected in the film deposited without the deliberate
addition of nitrogen again suggests that nitrogen exists in sufficient
quantities as a gas-phase impurity to influence the deposition process.
6.2.4 Secondary-Ion
Mass Spectroscopy (SIMS)
The use of SIMS was deemed necessary in the study of nitrogen incorporation into the films as prior Auger Electron spectroscopic studies proved too insensitive. Using a SIMS system, with operation in negative collection mode, mass spectra were collected for each sample. Figure 6.12 shows a typical mass spectrum of a film deposited with the inclusion of
1 sccm of nitrogen.

Figure 6.12
Negative Ion mass spectrum of film deposited with 1 sccm additional
nitrogen
The spectrum
clearly indicates a number of carbon clusters, also detectable are CN-
ions with a mass/charge of 26 Daltons.
In order to assess the quantity of nitrogen incorporated into the films
the ratio of CN- (26 Daltons) to C2- (24
Daltons) and C- (12 Daltons) was determined. Figures 6.13 and 6.14 show how the relation
of these ratios is dependent on the nitrogen added into the process gas.
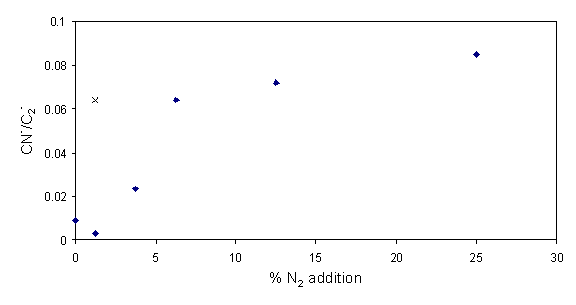 Figure 6.13 Ratio of CN-
to C2- from negative ion secondary ion mass spectra as a
function of additional nitrogen.
Figure 6.13 Ratio of CN-
to C2- from negative ion secondary ion mass spectra as a
function of additional nitrogen.
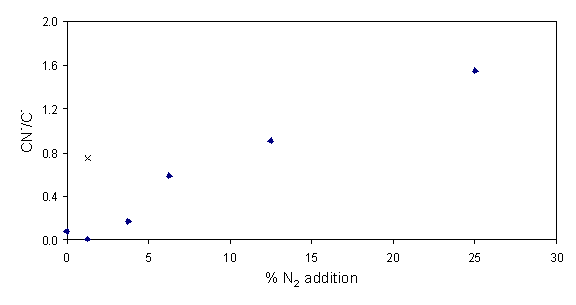
Figure 6.14 Equivalent plot to
figure 6.14 showing the ratio of CN- to C-.
Both trends clearly show nitrogen incorporation in the films increasing as a function of additional gas-phase nitrogen. Depth profiling of each sample showed no apparent change in nitrogen incorporation.
The centre of each film is considered to be of higher quality than the outer edges; a consequence of the radial difference in the gas-phase composition and the substrate temperature. The nitrogen incorporation in the more graphitic outer areas of a film deposited with 1 sccm added nitrogen were also investigated. The result of this investigation reveals increased nitrogen incorporation as shown in figures 6.13 and 6.14 (´). The increase in nitrogen incorporation may well derive from the fact that inclusion into a less packed density graphitic lattice carries a smaller energetic penalty than the equivalent addition to a diamond lattice.
6.3 Nitrogen-enhanced Growth Scheme
Growth schemes
for the enhanced deposition of diamond, with the addition of gas-phase nitrogen
have been proposed. Butler et al.[11]
have suggested a scheme for the enhancement of the nucleation rate on the
hydrogen terminated (111) diamond surface by addition of CN.
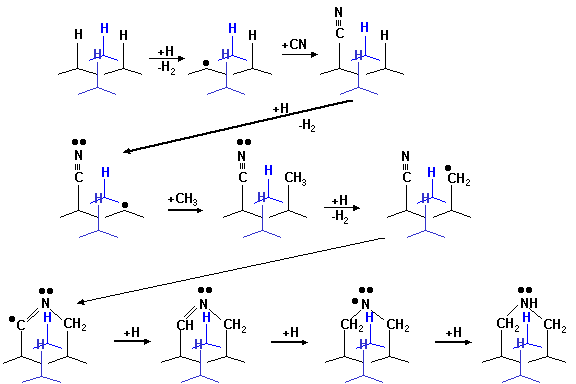
Figure 6.15
Growth scheme for the CN enhancement addition to (111) surface
Following
this, nucleation of the next layer is thought to occur via the scheme shown in
figure 6.16.
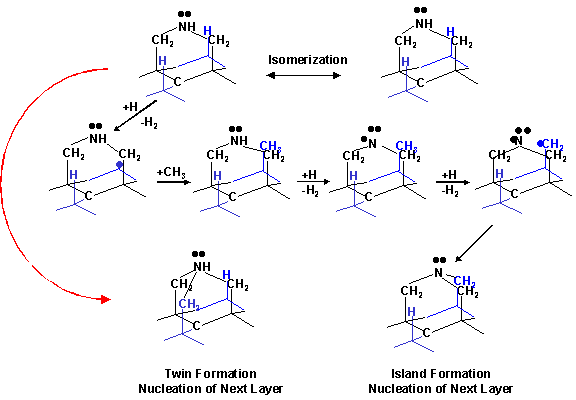
Figure
6.16 Growth scheme showing the next
layer nucleation on a (111) surface following that shown in figure 6.15.
These schemes
demonstrate that addition of nitrogen to the growing surface facilitates
nucleation of the next atomic layer, by providing a nucleation site, and thus
enhancing the growth rate of the diamond surface. While nitrogen incorporation at the growing surface encourages
the growth of both (100) and (111) facets, the (100) surface will be “grown
out” by the preferential and therefore more rapid growth of the (100) surface.
6.4 Conclusions
This chapter
has reported studies of diamond CVD at growth rates surpassing 100 mm hr-1
in a ≤10 kW DC-arc
jet system, operating with CH4/H2/Ar feedstock gas
mixtures. Controlled addition of N2
to the feedstock gas results in intense CN(B®X) emission and quenches C2(d®a) and Ha emissions
from the plasma. Abel transformation of
species selective, spatially resolved OES measurements has allowed derivation
of the longitudinal and lateral variation of emitting C2, CN and Ha species
within the plasma jet. SEM and laser
Raman analyses indicate that N2 additions also lead to a reduction
both in growth rate and quality of the resulting diamond film. The laser Raman, SIMS and PL measurements
also provide conclusive evidence of nitrogen incorporation (as chemically
bonded CN) in the films.