Chapter 1
Introduction
1.0 Outline
·
This
chapter outlines the history, properties and uses of natural and synthetic
diamond.
·
The
use of synthetic diamond as an electrochemical electrode is considered in terms
of the properties of the material.
·
The
existing literature on the electrochemistry of diamond is summarised.
·
The
chapter concludes with an brief summary of the work presented in this thesis.
1.1 The History of Diamond
Diamond has been valued for
its rarity for thousands of years. In the late 15th Century,
polishing techniques were developed to produce brilliant gems which utilised
the unique optical properties of diamond. In recent centuries, global
industries have developed which are involved with the mining, trading,
polishing and retailing of diamond gems. 1
The mining and processing of
natural diamond produces a large proportion of diamond that is insufficiently
aesthetically pleasing to be incorporated in to jewellery.†
A range of applications have been developed that put this by-product to practical
use. Applications include those that utilise its hardness (e.g. grinding,
cutting and polishing), its thermal properties (e.g. heat sinks) and its
optical properties.
Naturally occurring diamond
of terrestrial origin is formed at high temperatures and pressures in the
Earth’s mantle. Volcanic action is then responsible for carrying diamond into
the Earth’s crust. 3 Early finds of diamond were confined to
isolated crystals that had become exposed on the surface of the planet.
However, since the discovery of significant quantities of diamonds in South
Africa in the 1870s, industrial mining techniques have been developed. Diamond
remains a rare mineral but deposits have now been located at various locations
spread widely across the planet.
With the beginnings of
modern chemical studies in the late 18th Century came the first
indication of the composition of diamond. In 1796, Smithson Tennant studied the
combustion of diamond and showed it to be of the same composition as other
forms of carbon. 4
The confirmation of the
structure of diamond came from X-ray crystallography studies in the early part
of the twentieth century. 5 Figure 1.1 shows the structure and
symmetry of diamond.
1.2 Allotropes
of Carbon
There are two main
allotropes of carbon: diamond and graphite.
Diamond
·
sp3
bonding.
·
carbon
atoms forming tetrahedral bonds with four nearest neighbours.
·
crystals
with a cubic structure (see figure 1.1).
Graphite
·
sp2
bonding.
·
carbon
atoms forming three strong coplanar bonds with neighbouring atoms.
·
planes
of carbon atoms in a hexagonal arrangement.
·
unhybridised
2p orbital electron associated with each carbon atom creates p bonding between the neighbouring carbon
atoms in the plane (see figure 1.2).
·
only
weak bonding between the planar layers of atoms.
·
graphite
is the most common allotrope of carbon.
In 1985, a new class of
stable carbon molecules, known as fullerenes, was discovered. 6
They are based upon sp2 bonding configurations where the
introduction of larger or smaller rings of carbon atoms (heptagons and
pentagons) into a layer of graphite hexagons causes the structure to bend out
of plane. A vast number of interestingly shaped molecules are possible
including the highly symmetrical C60, other ball-like structures 6,7
and even tube-like structures. 8 The structure of C60
is represented in figure 1.3.
The different bonding in
diamond and graphite give the materials dramatically different properties. For
example, the strong rigid bonding in diamond makes the material mechanically
hard and electrically resistive. This contrasts with graphite, where the weak
interactions between graphitic planes make the material soft and the
delocalised p electrons make the material
electrically conductive in directions parallel to the plane.
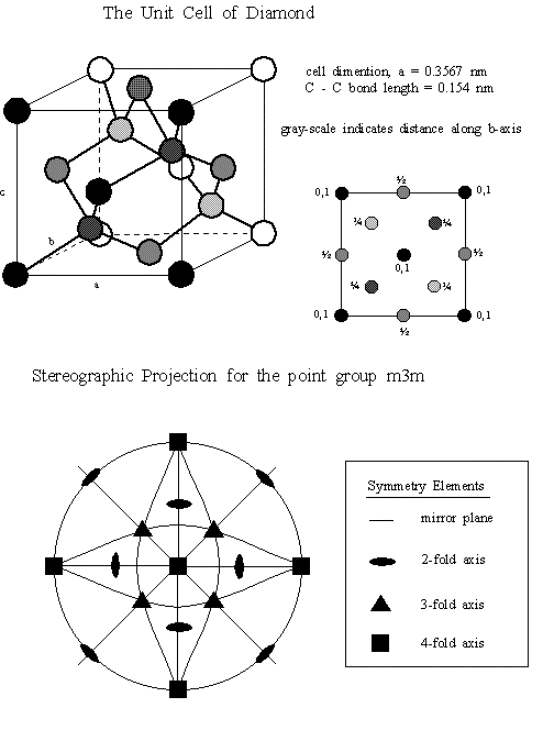
Figure 1.1
The structure of diamond

Figure 1.2
The structure of graphite
(reproduced from reference 9)
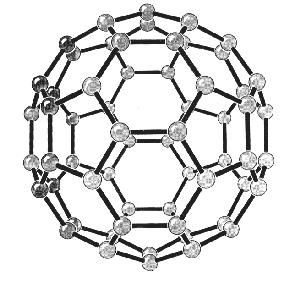
Figure 1.3
Buckminster Fullerene, C60
The very significant
differences between the properties of diamond and graphite are an important
consideration because small amounts of graphitic sp2 impurities in a
diamond crystal can dramatically alter the properties of the material.
In addition to crystalline
carbon, forms of amorphous carbon exist containing both sp2 and sp3
bonding in varying ratios. These materials often contain a significant
proportion of hydrogen in their structures. 10,11
Graphite is the
thermodynamically stable form of carbon at room temperature and pressure.
Diamond is only more thermodynamically stable than graphite at temperatures
greater than 1300 °C and pressures greater than
40 kilobar. There is only a small difference in the thermodynamic
stability of the two allotropes. At a temperature of 298 K and a pressure
of 1 standard atmosphere, the standard Gibbs free energy of formation of
diamond 12 is 2.9 kJ mol –1. A phase
diagram for carbon is shown in figure 1.4.
There is no easy
rearrangement mechanism by which diamond can convert to graphite. The energetic
activation barrier for conversion is very high and the conversion is therefore
kinetically unfavourable. Hence, diamond will remain in a meta-stable state at
room temperature and pressure without converting to graphite.
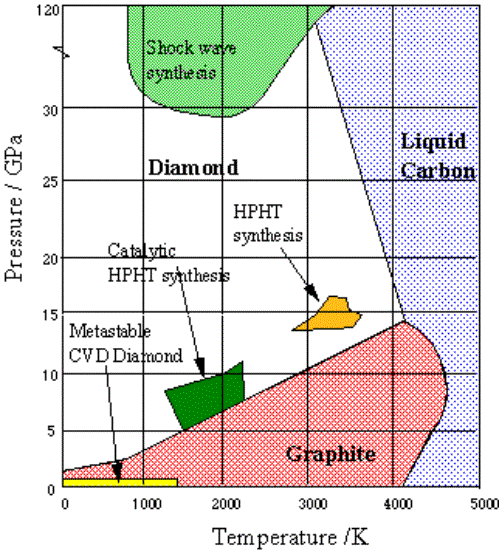
Figure 1.4
The phase diagram of carbon
(reproduced from reference
9)
1.3 Properties of Diamond
Diamond has a unique range
of extreme properties which have been reviewed extensively elsewhere.
5,13,14 A summary of properties is shown in Table 1.1.
A number of these properties
make diamond a promising choice of electrode material:
·
Diamond
has limited reactivity and may be used in a wide range of harsh environments.
·
Diamond
is chemically inert with respect to organic chemicals under normal conditions.
Diamond is therefore biologically compatible and so diamond electrodes may be
used as sensors in biological and medical experiments.
·
The
transparency of diamond over a broad spectral range allows for electrochemical
and spectroscopic studies to be combined. 15
·
The
doping of diamond with various concentrations of boron allows the electrical
properties of a device to be tailored.
·
CVD
diamond techniques allow thin layers of diamond to be deposited on to other
materials and therefore the material may be incorporated into electronic
devices.
·
Diamond
has a wide potential window which allows many electrochemical reactions to
occur (see section 1.6).
·
The
high thermal conductivity of diamond allows for greater temperature control at
the surface of the electrode.
·
Diamond
is radiation hard and devices have been fabricated that behave in a stable
manner while being irradiated. 16
Property Value
Hardness 1 ´ 10 4 kg mm -2
Tensile
Strength >
1.2 GPa
Compressive
Strength >
100 GPa
Dynamic
Coefficient of Friction 0.03
Sound
Velocity 1.8
´ 10 4 m s-1
Density 3.52 g cm-3
Young’s
Modulus 1.22 GPa
Poisson’s
Ratio 0.2
Thermal
Expansion Coefficient 1
´ 10 -6 K-1
Thermal
Conductivity 20.0 W cm-1 K-1
Thermal
Shock Parameter 3.0
´ 10 8 W m-1
Debye
Temperature 2200 K
Optical
Index of Refraction 2.41
at
591 nm
Optical Transmissivity 225
from
deep ultra-violet to far infra-red
Loss Tangent at 40 Hz 6.0 x
10 –4
Dielectric
Constant 5.7
Dielectric
Strength 1.0
´ 10 7 V cm –1
Electron
Mobility 2200 cm 2 V –1 s –
1
Hole
Mobility 1600 cm 2 V –1 s –1
Electron
Saturated Velocity 2.7
´ 10 7 cm s-1
Hole
Saturated Velocity 1.0
´ 10 7 cm s-1
Work
Function on [111] surface “negative”
Bandgap 5.45
eV
Resistivity
of undoped diamond ~
10 16 W cm
Heat
Capacity 6.195 J mol-1 K-1
Boron
Diffusivity at 1073 K 6.9
´ 10 –20 cm2 s-1
Table 1.1
Selected properties of
diamond
(figures are for single
crystal diamond)
(values measured or
calculated at room temperature unless otherwise stated)
1.4 The Synthesis of Diamond
Early experiments to produce
synthetic diamonds used a high pressure, high temperature (HPHT) process that
produced diamond under conditions where it was the thermodynamically stable
form of carbon. This technique was pioneered by the General Electric company of
America in 1955. 13 HPHT
grown diamond tends to consist of small crystals of a few millimetres in
diameter.
Chemical vapour deposition
(CVD) is a process that can be used to synthesise diamond at much lower
temperatures and pressures under non-equilibrium conditions. The technique is
now established and has been well described in a number of review articles. 11,17,18
Chapter 2 outlines the CVD
process used in these studies.
1.5
Uses of Diamond
Many applications of diamond
that make use of the extreme physical properties of the material are in use or
have been proposed. 19 A number of these applications are outlined
below:
1.5.1 Abrasives
Existing uses of natural and
HPHT diamond include abrasion, cutting and polishing. These applications
exploit a number of the properties of the material including: hardness, thermal
conductivity, lack of chemical reactivity and the low coefficient of friction
for hydrogen terminated diamond. The high cost of diamond restricts its use to
heavy duty industrial tools, such as diamond saws and precision tools, such as
scalpels and dental drills.
CVD techniques can be used
to coat diamond onto carbide forming metals but widespread use of diamond
coated tools is prevented by the mismatch in the thermal expansion coefficients
of diamond and the underlying metals. Recent studies have investigated the
feasibility of reducing the temperature required for CVD diamond deposition. A
reduction in the deposition temperature may widen the range of materials that
may be coated and permit a greater number of diamond tools to be produced.
CVD diamond has been used to
replace natural diamond in a number of applications where control of the
geometry can improve performance of the device. For example, a range of
scalpels specifically designed for eye surgery have been produced. 20
While the hardness of
diamond has not yet been utilised in cutting tools produced for the mass
market, hard amorphous carbon films, which can be deposited at room temperature
have been developed for use on razor blades. 21 †
1.5.2 Thermal Management
Diamond has a very high
thermal conductivity and it is an electrical insulator. This makes it a
suitable material for thermal management applications. The use of diamond as a
heat sink for integrated circuits has the potential to allow closer packing of
features and lower operating temperatures. Diamond is currently used as a heat
sink for specialist applications such as semiconductor laser diodes, microwave
diodes and small microwave integrated circuits. 14,22
1.5.3 Electronic Devices
The extreme properties of
diamond would be of great benefit if the material could be incorporated into
electronic devices. Undoped diamond is an electrical insulator with a
wide-bandgap (5.45 eV) but the electrical properties of diamond, like other
group IV elements, may be altered by the addition of impurities.
![]()
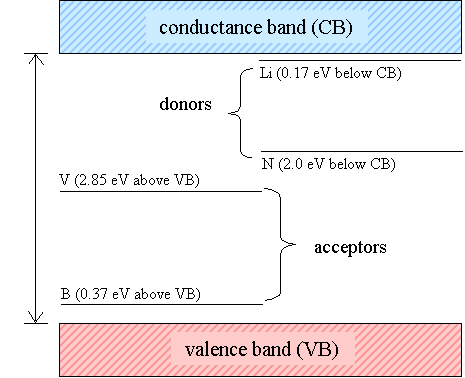
Key
B = boron
N = nitrogen
Li = lithium
V = vacancy
Figure 1.5
An energy diagram of
selected states in the band gap of diamond
(one dimensional
representation)
Boron doping can generate
p-type behaviour in the material. The substitutional boron dopant atoms form an
acceptor levels at ~ 0.35 eV above the valence band as shown if figure 1.5.
5 At low doping levels, the diamond acts as an extrinsic semiconductor.
At high doping levels the material acts as a semi-metal. 23,24
Diamond has a close packed
rigid structure which does not readily accept substitutional impurity atoms.
Doping with phosphorous and arsenic has not successfully generated n-type dope
diamond. Current research is investigating the electrical properties of sulphur
doped diamond. 25,26
The use of CVD diamond films
for semiconductor devices is hampered by the polycrystalline nature of the
films. The numerous grain boundaries and crystal defects reduce electron and
hole mobilities and generally degrade electronic performance. Therefore only
limited success has been achieved the production of electronic devices. 27
Greater success has been achieved in fabricating sensors 16,28
and electrodes. 29
The low or negative electron
affinity that is observed in diamond is a property that it may be possible to
exploit in field emission displays. 30,31
1.5.4 Optical Windows
Diamond has a broad band
transparency and is across the visible, infra-red and ultra-violet regions of
the spectrum. In particular, diamond is transparent in the wavelength range
from 8 mm to 12 mm. This corresponds to an atmospheric
“window” where there is no significant absorption of infra-red radiation by
molecules in the atmosphere.
Many applications, such as
thermal imaging, require infra-red windows. Many common materials used for
these windows have drawbacks to their use. For example, zinc sulphide (ZnS) is
too soft to be used in hostile environments. CVD diamond can be used to coat
infra-red windows to give then a hard, thermally conductive outer layer.
Alternatively, free standing CVD grown diamond films could be used as a wide
spectrum window giving transparency over a range of wavelengths.
There are currently a number
of technical problems that must be overcome before the optical properties of
diamond can be fully exploited:
·
Growth
of CVD diamond on planar substrates is well established. Growth of diamond onto
substrates with more interesting geometries, such as convex surfaces, is less
common. Many optical applications require non-planar shapes.
·
CVD
grown diamond has a rough top surface due to the polycrystalline nature of the
films. Optical applications require smooth surfaces and the grinding of diamond
to smooth its surface is problematic due to its extreme hardness.
·
Impurities
and defects in the crystals alter the optical properties of diamond. The common
classification of natural diamond as Class I or Class II was established in
1934 and was based upon transparency to 8 mm infra-red radiation.
5 It is now known that nitrogen
impurities are responsible for making Class I diamond opaque to 8 mm radiation. Low concentrations of impurities
can be tolerated but most applications require constant predicable performance.
It is therefore important to ensure that any impurities are at low levels,
evenly distributed and in known forms.
·
The
reactivity of diamond in hot, oxygen containing environments limits the use of
diamond in the aerospace industry.
·
Diamond
films can too be brittle for practical use.
1.6 The Electrochemistry of
Boron Doped Diamond
Carbon based electrode
materials are well established and have been used in varied electrochemical
technologies from electroanalysis to energy storage. 23 The
structure of existing electrode materials varies but they are all based upon
graphitic (sp2) bonding schemes, for example: glassy carbon,
graphite, carbon fibres.
As summarised in section
1.3, diamond possesses a range of properties that may make it useful for
electrochemical applications. The advent of relatively inexpensive CVD diamond
techniques has generated great interest in the electrochemical applications of
diamond. 29
The first electrochemical
experiments on CVD diamond were published in 1987 32 but work
was hampered by poor understanding and characterisation of the non-diamond
carbon and dopant atom content of the films. Early experiments performed with
boron doped diamond electrodes studied heavily doped samples that gave
promising results. Xu et al. (1997) 23
published a review of the early results which is summarised below:
·
low
background currents
(an order of magnitude less than for glassy carbon)
·
low
double layer capacitance
(an order of magnitude less than for glassy carbon)
·
featureless
response in 0.1 M KCl from -1000 mV to 1000 mV
(current density < 50 mV/cm2)
·
wide
potential window 33
(large overpotential for the evolution of chorine, oxygen and hydrogen)
Three possible factors that
may explain the behaviour are given:
1.
an
absence of electroactive carbon-oxygen functionalities on the
hydrogen-terminated surface.
2.
a
low density of surface electronic states near the Fermi level caused by the
semimetal-semiconductor nature of boron doped diamond.
3.
the
diamond surface acts as an array of microelectrodes with many separate
“electrochemically active” regions separated by more insulating regions.
The possibility of an inert
working electrode with a wide potential range with a low background current has
generated a number of papers outlining possible uses for diamond electrodes. A
selection of the available literature is listed in table 1.2.
First Named Author & Year |
Ref |
Title |
|
Tenne, R. (1993) |
34 |
Efficient
electrochemical reduction of nitrate to ammonia using conductive diamond film
electrodes |
|
Katsuki, N. (1997) |
35 |
Electrolysis by using
diamond thin film electrodes |
|
Beck, F. (1998) |
36 |
Boron
doped diamond/titanium composite electrodes for electrochemical gas
generation from aqueous electrolytes |
|
Bouamrane, F. (1998) |
37 |
Underpotential
deposition of Cu on boron-doped diamond thin films |
|
Compton, R. G. (1998) |
38† |
Sonoelectrochemical
production of hydrogen peroxide at polished boron-doped diamond electrodes |
|
Katsuki, N. (1998) |
40 |
Water
electrolysis using diamond thin-film electrodes |
|
Yano, T. (1998) |
41 |
Electrochemical
behavior of highly conductive boron-doped diamond electrodes for oxygen
reduction in alkaline solution |
|
Agra-Gutiérrez, C. (1999) |
42 |
Anodic stripping
voltammetry of copper at insonated glassy carbon-based electrodes:
application to the determination of copper in beer |
Table 1.2a
A sample of the literature
on the applications of diamond electrochemistry
First Named Author & Year |
Ref |
Title |
|
Fujishima, A. (1999) |
43 |
Electroanalysis
of dopamine and NADH at conductive diamond electrodes |
|
Granger, M. C. (1999) |
44 |
Polycrystalline
diamond electrodes: basic properties and applications as amperometric
detectors in flow injection analysis and liquid chromatography |
|
Manivannan, A. (1999) |
45 |
Detection
of trace lead at boron-doped diamond electrodes by anodic stripping analysis |
|
Nakabayashi, S. (1999) |
46 |
Dye
sensitization of synthetic p-type diamond electrode |
|
Okino, F. (1999) |
47 |
Electrochemical
fluorination of 1,4-difluorobenzene using boron-doped diamond thin-film
electrodes |
|
Popa, E (1999) |
48 |
Selective
electrochemical detection of dopamine in the presence of ascorbic acid at
anodized diamond thin film electrodes |
|
Saterlay, A. J. (1999) |
49 |
Sono-cathodic stripping
voltammetry of manganese at a polished boron-doped diamond electrode:
application to the determination of manganese in instant tea |
|
Vinokur, N. (1999) |
50 |
Cathoidic
and anodic deposition of mercury and silver at boron-doped diamond electrodes |
|
Yano, T. (1999) |
51 |
Electrochemical
behavior of highly conductive boron-doped diamond electrodes for oxygen reduction
in acid solution |
|
Yoshihara, S. (1999) |
52 |
Photoelectrodeposition of
copper on boron-doped diamond films: application to conductive pattern
formation on diamond. The photographic diamond surface phenomenon |
|
Beck, F. (2000) |
53 |
Boron
doped diamond (BDD)-layers on titanium substrates as electrodes in applied
electrochemistry (paper briefly reviews waste water treatment and electro-synthesis) |
|
Fujishima, A. (2000) |
54 |
TiO2
photocatalysts and diamond electrodes (paper
describes the detection of histamine, the detection of the coenzyme NADH and
the trace analysis of lead) |
|
Michaud, P. -A. (2000) |
55 |
Preparation of peroxodisulfuric acid using
boron-doped diamond thin film electrodes |
|
Rao, T. N. (2000) |
56 |
Recent
advances in electrochemistry of diamond (paper
describes the detection of histamine and sulfadiazine) |
|
Wang, J. (2000) |
57 |
Incorporation
of Pt particles in boron-doped diamond thin films: Applications in
electrocatalysis (considers the underpotential deposition of hydrogen and oxidation of methanol) |
Table 1.2b
A sample of the literature
on the applications of diamond electrochemistry
Despite the widespread
attempts to utilise diamond for electrochemical applications, the mechanism of
the electron transfer across the diamond/electrolyte interface still needs to
be determined.
Early experiments
investigating the cyclic voltammetry and AC impedance analysis of boron
doped diamond used samples with uncontrolled or undisclosed levels of boron
doping and focused on the effects of non-diamond content in the films. 58,59
AC impedance studies of
highly doped single crystal diamond (homoepitaxial layers grown on natural
diamond) have shown that the charge transfer was mediated by undetermined
surface states. 60
1.6
Summary
Diamond is a unique material
with many extreme properties. The advent of reliable, inexpensive methods of
synthesis could lead to the use of diamond in a wide range of applications.
Pure diamond is a very good electrical insulator but the material may be doped
with boron to produce electrodes with semiconducting or semimetallic
properties. The use of diamond electrodes has been proposed for many
electrochemical applications. The existing literature focuses on highly doped
electrodes and does not fully explain the mechanism of the electron transfer across
the diamond/electrolyte interface.
1.7 Outline of the Thesis
Table 1.3 summarises the
contents of the chapters in this thesis.
Chapter 1 Introduction |
· This chapter outlines the
history, properties and uses of natural and synthetic diamond. · The use of synthetic
diamond as an electrochemical electrode is considered in terms of the
properties of the material. · The existing literature on
the electrochemistry of diamond is summarised. · The chapter concludes with
an brief summary of the work presented in this thesis. |
Chapter 2 Growth |
· This chapter describes the
diamond growth method used in this study and explains why it was chosen. · The main components of the
diamond growth apparatus are described. · The benefits of four
different types of electrical contact are compared. · Scanning electron
microscopy, optical microscopy and Raman spectroscopy were used to
characterise the films and the results are presented here. |
|
Chapter 3 Electrical Contacts |
· This chapter describes the
electrical contacts between the diamond electrodes and the copper connecting
wires. · The requirement for Ohmic
contacts is discussed. · Details are given for the
fabrication processes for two types of Ohmic contact: 3LM contacts and TiUL contacts. The practicality of the two
processes for use in the laboratory are compared. · Current-voltage plots are
presented for the four types of contacts described in chapter 2. A comparison
is made between the performance of the four types of contact. |
|
Chapter 4 Standard Electrochemical Theory |
· This chapter outlines the
standard electrochemical theory on which this study was based. · A comparison is made
between the behaviour of metal and semiconductor electrodes. · An expression is derived
relating the steady state current to the overpotential. |
Chapter 5 The Electrochemistry of Highly Doped Diamond Films |
· This chapter contains
details of the apparatus used for the electrochemical experiments in these
studies. · The chapter describes the
electrochemistry of highly doped diamond films. Cyclic Voltammogams were
recorded with a number of well known redox couples to show the behaviour of
the films. · The effect of surface
termination was investigated. · AC Impedance and
Mott-Schottky plots are presented. |
Chapter 6 The Electrochemistry of Low Doped Diamond Films |
· The chapter describes the
electrochemistry of low doped diamond films. Cyclic Voltammogams are
presented for a number of well known redox couples. · The effect of surface
termination of the electrode was investigated. · The effect of the concentration
of the electroactive species was studied. · The results of the
electrochemical experiments are explained in terms of the theory outlined in
chapters 4 and 7. |
Chapter 7Theoretical Model for the Electrochemistry of Boron Doped Diamond |
· This chapter develops a
theoretical model for the electrochemistry of boron doped diamond. · The relationship between
current density and applied potential is investigated · The AC impedance results
are considered in terms of the surface state model. |
|
Chapter 8 Intensity
Modulated Photocurrent Spectroscopy |
· This chapter presents the
results of Intensity Modulated Photocurrent Spectroscopy (IMPS) experiments
on boron doped diamond films. · A theory is presented to
explain the results. |
|
Chapter 9 Conclusions |
· The main results of the
research are summarised. · Suggestions for possible
future work are presented. |
Table 1.3 - outline of the chapters in this thesis