Chapter 4 - Results for C/H Mixtures
“A posse ad esse”
4.1 Introduction
This chapter presents the results of MWCVD diamond growth experiments using hydrocarbon/H2 source gas mixtures. Each section describes a single CVD property (gas composition, microwave power, pressure etc.) whose effect on diamond film growth and gas phase composition (determined by MBMS) was investigated. The traditional CH4/H2 CVD gas mixture was used for these experiments, and in many cases C2H2/H2 and C2H4/H2 gas mixtures were also studied. A summary of the experiments and their respective sections are given in Table 4.1. Each section will begin with details of the growth experiments performed. Data will be presented in the form of graphs showing film growth rates, and morphological characteristics, as judged by SEM. Laser Raman spectra will also be presented, where necessary, to indicate the quality of the deposit. Where SEM or Raman data becomes too lengthy or cumbersome to fit succinctly into this chapter, reference will be made to the appropriate pages in Appendix A where the interested reader may find the data in full. Finally, where applicable, MBMS studies of the gas-phase composition under identical conditions to those used for the film deposition will be presented. Each section will conclude with a discussion pertinent to the results presented therein.
|
Chapter/
Section |
Gas(es)
added to H2 |
Chemical
Formula |
Deposition
Runs? |
MBMS
Gas-Phase Analysis? |
|
4.3-4.9 |
Methane |
CH4 |
ü |
ü |
|
4.4-4.6 |
Acetylene |
C2H2 |
ü |
ü |
|
4.4-4.6 |
Ethylene |
C2H4 |
ü |
ü |
|
5 |
Argon |
Ar |
N/A |
ü (temperature measurements) |
|
6.2.1, 6.3.1 |
Methane/ Nitrogen |
CH4/N2 |
ü |
ü |
|
6.2.2, 6.3.2 |
Methane/ Ammonia |
CH4/NH3 |
ü |
ü |
|
6.2.3 |
Methylamine |
CH3NH2 |
ü |
ü |
|
6.2.4 |
Hydrogen Cyanide |
HCN |
û |
ü |
Table 4.1 Summary of experiments performed in this thesis.
4.2 Experimental Considerations
The dilute CH4/H2 gas mixture forms the most commonly used CVD diamond growth ‘recipe’. It’s origins lie in the pioneering work at NIRIM in Japan where this process gas mixture was first successfully used for low pressure CVD diamond growth (Section 1.3) [[1],[2]]. When the present project was initially undertaken, only a small amount of work had been performed characterising the diamond films grown using our MWCVD reactor with a CH4/H2 feed [[3]]. Thus the CVD reactor parameters - Microwave power, pressure, CH4 percentage in H2, and substrate temperature were all varied in order to examine their effect on the deposit obtained during CVD diamond growth. Initially, diamond films were grown using the old, larger diameter substrate heater. This was replaced with a smaller diameter substrate holder (to eliminate secondary plasmas, Section 3.2.8.1) prior to starting the mass spectrometric work. As stressed in Section 1.4, the internal geometry of the reactor is critical to the operation of the microwave plasma reactor. We could not be sure a priori what the effect of a new substrate holder would be, so a certain amount of growth experiment repetition was necessary to compare films grown using the two different reactor geometries. Therefore many of the growth rate graphs will have two curves - one curve for each diameter of substrate holder. SEM images will also be increased in number. All MBMS experiments were carried out using the new substrate holder, as this was the only way we could guarantee the absence of secondary plasma discharges in the reactor, and thus reproducible conditions.
Experimentally, it was found that when using the new substrate holder, the temperature controller had to be set to 700°C to cause it to glow with a similar dull red colour to the larger, old substrate holder (when set at 800°C). This was the only means of comparing the substrate temperature using the two substrate holders, as we did not have access to a pyrometer suitable for temperature measurement in the 500-1000°C range. The colour of the substrate holder at 650°C and 750°C using the new substrate holder was noticeably different from that seen when using the old substrate holder set at 800°C. We therefore believe that identical substrate temperatures (judged visually) of the old and new substrate holders led to thermocouple measurements differing by (100±30) K between the old and new substrate holders. This is probably due to the different size and geometry of the two substrate holders. The position of the thermocouple relative to the substrate is also slightly different in the new substrate holder.
4.3 Effect of Deposition Time
A diamond film was grown over many days in ~6 hour periods, utilising identical growth conditions each day. These conditions were typical for diamond growth using our microwave plasma facility - 1000 W microwave power, 1.44 sccm CH4/200 sccm H2 (0.72% CH4/H2) at a pressure of 30 Torr, and 800°C substrate temperature (old substrate holder). ‘sccm’ refers to the gas flow in units of standard centimetres cubed per minute. At the end of each days growth, a small section of the substrate was broken off, and its thickness and morphology examined by SEM. The evolution of film thickness with time is shown in Fig. 4.1.
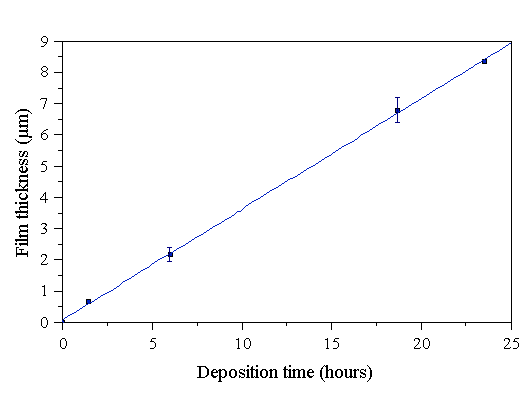
Figure 4.1 Evolution of diamond film thickness with time. Deposition conditions: 1000 W microwave power, 30 Torr pressure, 0.72% CH4/H2, 202 sccm total gas flow, using the old substrate holder, set at 800°C.
It can be seen that the thickness of the film increases linearly with time. There is no incubation period prior to film growth, as was found to be the case on Si wafers with a low density of nucleation sites prior to growth (i.e. no manual abrasion) [[4],[5]]. It is therefore straightforward to determine the growth rate, which is simply the gradient of Fig. 4.1, whose numerical value is (0.36±0.01) mm h-1. Further, these data show that the growth rate of an individual film can be accurately determined, simply by dividing the film thickness by the growth time, which is how all subsequent growth rates in this thesis have been determined. The morphology changes occurring over time during growth are shown in Figs. A.1-A.4 (Appendix A). It can be seen that early in the growth, crystal facets are well defined until the individual diamond particles coalesce. After this occurs, the crystal facets become larger until secondary nucleation of many small diamond crystals occurs after ~5-6 hours growth, obscuring the faceting. The appearance of secondary nucleation is typical of all films grown for ~6 hours. After this time the secondary nucleated crystals grow, developing larger, smooth facets. Such re-nucleation was noted in the study of diamond films grown by Kobashi et al using MWCVD [[6]]. Also, as will be noted in later sections, films grown with the old substrate holder appear to be more finely grained, and less well faceted than those grown with the new substrate holder.
4.4 Effect of Hydrocarbon Mole Fraction in H2
4.4.1 Growth Rates
Diamond films were deposited using a range of CH4 input mole fractions. The variation of the linear growth rate, determined by the method of the previous section is shown in Fig. 4.2.
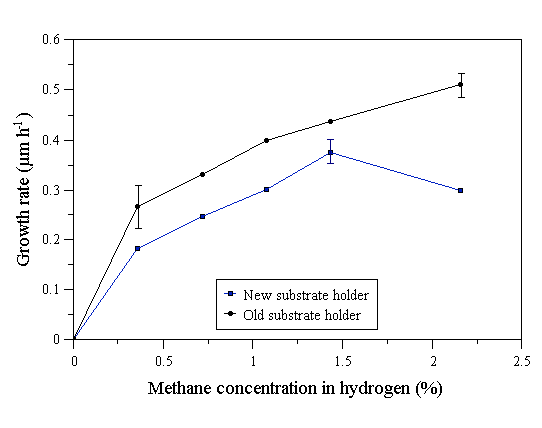
Figure 4.2 Growth rate of diamond films grown by MWCVD as a function of input CH4 mole fraction. 1000 W microwave power, 30 Torr pressure, 200 sccm total gas flow rate, substrate temperature 800°C (old substrate holder) or 700°C (new substrate holder).
It can be seen that the growth rate increases with input CH4 mole fraction. Similar behaviour was previously reported by Inspektor et al [[7],[8]] who found the growth rate to plateau at ~3% CH4, and then to decrease at higher input CH4 mole fraction. The growth rate using the old substrate heater is greater than that obtained with the new substrate heater by a factor of ~1.3, however, the same behaviour is seen with respect to changing CH4 mole fraction. The morphological variation of the films with increasing CH4 mole fraction (Figs. A.5-A.15) is the same as that seen by Kobashi et al [6], namely that there is a change from (111) to (100) to nanocrystalline film texture as the input CH4 mole fraction increases. This is less evident for the films grown with the old substrate heater (Figs. A.5-A.10), which show poor faceting and small crystal size. It would seem that the elimination of secondary plasmas is a necessary condition for the growth of well faceted diamond films in the microwave plasma system.
4.4.2 Film
Quality
The Raman spectra of diamond films grown with increasing input CH4 mole fraction (samples A-E) were examined using four different laser excitation wavelengths, and the results of this section have been published in Diamond & Related Materials [[9]]. These data are reproduced in Fig. 4.3 overleaf. Growth conditions for samples A-E were: 1000 W microwave power, 800°C substrate temperature, 30 Torr pressure, ~200 sccm total gas flow, using the old substrate holder. The input CH4 mole fractions are given below in Table 4.2.
|
Sample |
A |
B |
C |
D |
E |
|
Input CH4
mole fraction (%) |
0.36 |
0.72 |
1.08 |
1.44 |
2.16 |
Table 4.2 Input CH4 mole fractions for diamond film samples A-E.
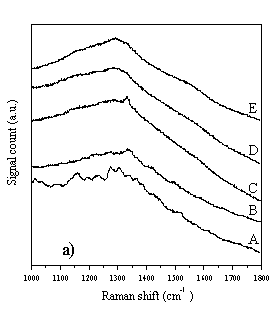
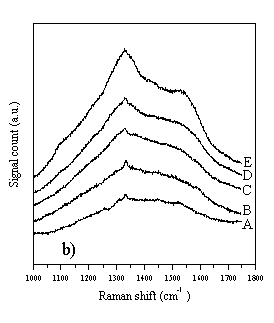
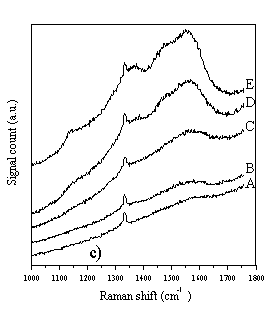
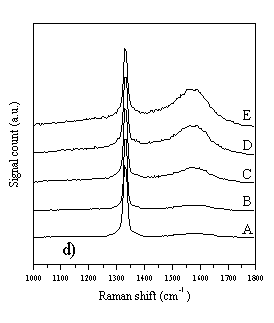
Figure 4.3 Raman spectra of diamond films A-E, taken with a variety of different wavelength laser excitations. Figure 4.3a) Raman spectra taken using 780 nm diode laser (near IR) excitation and a Renishaw System 2000 Raman spectrometer. Figure 4.3b) As 4.3a, except 633 nm HeNe laser excitation. Figure 4.3c) As 4.3a, except 514 nm Ar+ laser excitation. Figure 4.3d) As 4.3a, except 244 nm (UV, frequency doubled 488 nm Ar+) laser excitation.
It can be seen from Fig. 4.3 that the choice of wavelength used for Raman studies strongly affects the appearance of the Raman spectrum. IR laser wavelengths are sensitive to the Raman features of the non-diamond components in the film, but scarcely register the diamond peak at 1332 cm-1. At the other extreme, Raman spectra obtained using UV laser wavelengths selectively reveal the diamond peak, but display little sensitivity toward the non-diamond components of the film. This changing sensitivity with laser wavelength has been observed previously [[10]-[14]], but not in a set of CVD diamond films grown systematically whilst varying the input CH4 mole fraction, and not over such a comprehensive range of wavelengths (for CVD diamond). These observations have been attributed to resonance effects [12,[15]], or to an increased absorption of laser radiation by non-diamond components of the film with decreasing wavelength [10,14]. In addition to the main diamond peak at 1332 cm-1 these spectra also reveal the G- and D-bands of microcrystalline graphite at ~1580 cm-1 and ~1350 cm-1 respectively [[16],[17]]. The G- and D-bands become more prominent, relative to the diamond peak, with increasing input CH4 mole fraction (sample A®E), indicating that more graphitic material is deposited at higher input CH4 mole fraction. As noted in Chapter 2, it is very difficult to extract meaningful quantities from such data (such as sp3:sp2 ratios), and this has not been attempted in this study. Amorphous carbon produces a broad, asymmetric Raman peak between 1000-1600 cm-1 [[18]], peaking around 1450 cm-1, but this feature does not appear to contribute significantly to the data of Fig. 4.3. Since the Raman cross-section for amorphous carbon is much larger than that of diamond [[19]], the absence of a prominent Raman feature at 1450 cm-1 implies that the amount of amorphous carbon incorporated into the film is very small, compared with the other carbonaceous components. Therefore, the films A-E are best considered as consisting almost entirely of diamond, with microcrystalline graphitic inclusions. Further evidence in support of this hypothesis can be found when the wavelength dependence of the individual Raman peaks is examined. The spectra in Fig. 4.3a-d were curve fitted using Renishaw System 2000 Raman spectrometer data analysis software. This allowed the Stokes shift of each Raman peak to be determined, for each of the four laser wavelengths used in this study. The results are plotted in Fig. 4.4.
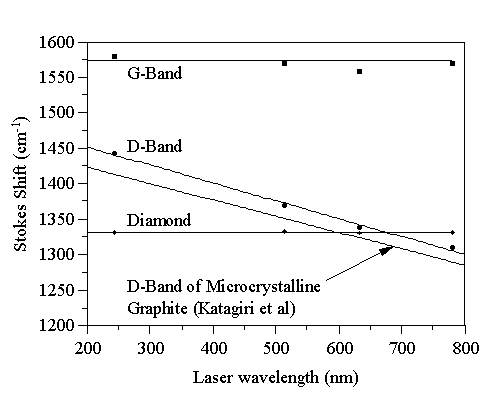
Figure 4.4 Wavenumber positions of the major CVD diamond Raman peaks.
Fig. 4.4 shows that the wavenumber position of the diamond peak and G-band remain constant with increasing laser wavelength (at 1332 cm-1 and 1580 cm-1, respectively), whereas the D-band shifts down in frequency. This behaviour has been observed in microcrystalline graphite [[20]], and diamond-like carbon (DLC) films [18], but has not yet been satisfactorily explained. The trend-line of Katagiri et al [20] for microcrystalline graphite has been included in Fig. 4.4, and our results lie close to this line, with a similar gradient, supporting the view that the principal non-diamond component in our CVD diamond films is microcrystalline graphite.
4.4.3 MBMS Gas
Phase Studies
MBMS studies of the gas phase composition during MWCVD whilst varying the input CH4 mole fraction are presented below. Firstly, cracking patterns were recorded for the expected major stable hydrocarbon species (CH4, C2H2, C2H4) to determine the electron energies that needed to be set to detect each species, whilst minimising interference from possible cracking products. A cracking pattern for C2H4 has already been shown in Fig. 3.13. Cracking patterns for CH4 and C2H2 are shown in Figs. 4.5 and 4.6, respectively. Sampling probe 1 (described in Section 3.3.1) was used for all mass spectrometric work in this thesis except where explicitly stated otherwise.
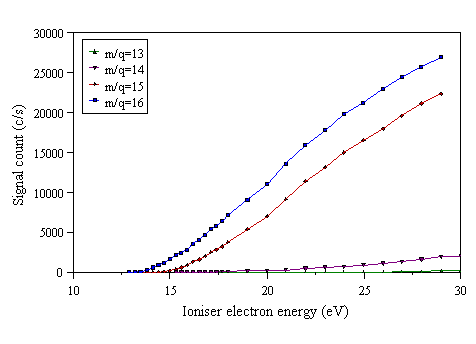
Figure 4.5 Cracking pattern of CH4.
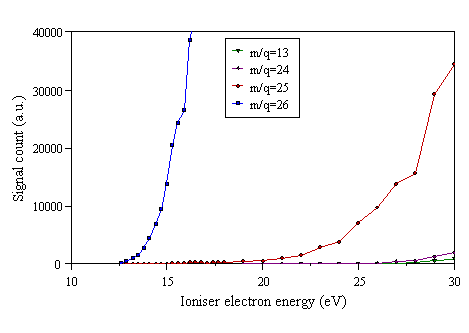
Figure 4.6 Cracking pattern of C2H2.
The appearance potentials of possible cracking products were determined from these cracking patterns (Table 4.2). From these data, the electron energies used to detect each hydrocarbon species were chosen. For all mass spectrometric data in this chapter, the electron ioniser energies used were: H2 and CH4:16.0 eV, C2H2:13.2 eV (to minimise signal due to cracking from C2H4 occurring above 13.5 eV), C2H4:11.9 eV (to avoid possible detection of residual N2 above 15.5 eV, and CO above 14.0 eV), and CH3:13.6 eV (to reduce signal due to cracking from CH4 above 14.3 eV).
|
|
CH4+ |
CH3+ |
CH2+ |
CH+ |
C2H4+ |
C2H3+ |
C2H2+ |
C2H+ |
|
CH4 |
13.1 13.0 |
14.3 15.0 |
15.6 16.0 |
23.0 24.0±1 |
|
|
|
|
|
C2H2 |
|
|
|
22.3 25±2 |
|
|
11.4 13.5 |
17.8±0.5 20.5±2 |
|
C2H4 |
|
|
19.3 |
23.0 |
10.5 10.5±0.3 |
14.0 14.6 |
13.5 13.5±0.3 |
19.3 |
Table 4.2 Measured ionisation potentials of CH4, C2H2, C2H4 and appearance potentials of their cracking products (all in eV). Literature values [[21]] are in italics, with experimentally measured values underneath. Measured values are accurate to ±0.5 eV, except where otherwise stated. Adapted from [[22]].
MBMS measurements were taken under conditions of 300, 500, and 1000 W applied microwave power, and are presented on the following pages (Figs. 4.7-4.9). These demonstrate the effect of varying the input CH4 mole fraction. The mole fraction data on the vertical axis of these graphs, and subsequent MBMS results, were corrected from the signal data and calibrated according to the procedure laid down in Sections 3.7 and 3.8. An important point to remember (from Fig. 3.18) is that the level of applied microwave power changes the size and location of the plasma ball. At low powers (~300 W) a point close to, but just inside, the visible plasma edge is sampled. At high powers (~1000 W) a point deeper in the plasma is sampled. Typical errors in the MBMS data in this chapter are ~±5% for CH4 and C2H2, ~±50% for CH3, and ±20% for C2H4, which are difficult to indicate easily on many of these (MBMS results) graphs.
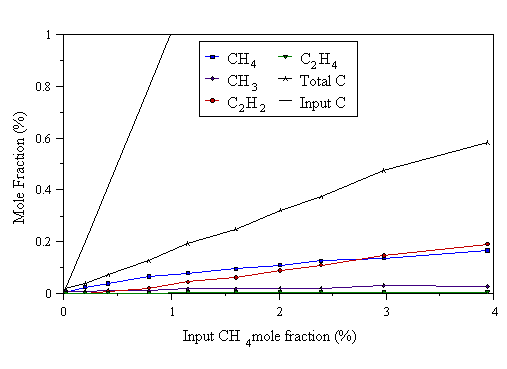
Figure 4.7 MBMS data taken from a 1000 W CH4/H2 microwave plasma. 700°C substrate temperature, 20 Torr pressure.
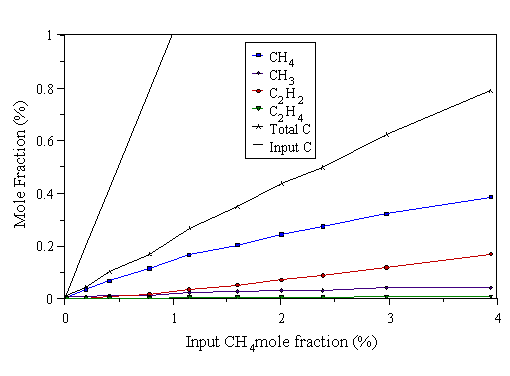
Figure 4.8 MBMS data taken from a 500 W CH4/H2 microwave plasma. 700°C substrate temperature, 20 Torr pressure.
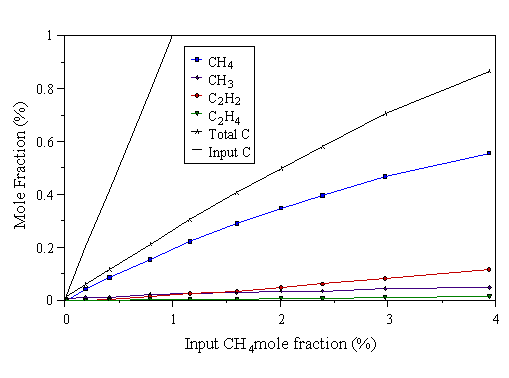
Figure 4.9 MBMS data taken from a 300 W CH4/H2 microwave plasma. 700°C substrate temperature, 20 Torr pressure.
The first point to note on inspecting Figs. 4.7-4.9, is that the measured carbon total falls short of the input carbon level. This drop in total carbon becomes more pronounced at higher microwave powers (better illustrated by the data in Section 4.5). We believe this to be due to mass dependent thermal diffusion causing hydrocarbon species in the plasma centre to preferentially diffuse away from the hot regions of the plasma to the cooler surroundings, ‘down’ a temperature gradient. Evidence for temperature gradients in the plasma - necessary for thermal diffusion to occur - will be presented in Chapter 5. In all three graphs, at low input CH4 mole fraction, CH4 is the most prevalent hydrocarbon species in the plasma. CH3 is the next most abundant hydrocarbon at low input CH4 mole fraction. This corresponds to the conditions where the highest quality CVD diamond is grown, as evidenced by the laser Raman data presented in Section 4.4.2 above, and in [9]. Very little C2H2 is detected at low input CH4 mole fractions. At higher input CH4 mole fractions, the measured C2H2 mole fraction increases. Thus, increasing the input CH4 increases the fraction of C2 species in the gas phase, relative to C1 species. This should be contrasted with HFCVD, where it was found that CH4 was the most prevalent gas-phase hydrocarbon at high input CH4 mole fraction [[23]]. In the hot filament reactor, dissociation of H2 is believed to occur primarily at the filament surface. At high input carbon levels, the filament surface becomes saturated with hydrocarbon species, hindering the dissociation of H2, which inhibits the usual H-driven CVD gas-phase chemistry. The evidence that we do not observe such ‘filament saturation’ effects in the MWCVD system (Figs. 4.7-4.9) is consistent with the notion that dissociation occurs throughout the plasma.
The Raman data shown in Section 4.4.2 indicate that the poorest quality deposits are formed at high input CH4 mole fraction. Taken with the MBMS data, these observations agree with the results of Mitsuda et al [[24]], who found that the existence of intense optical emission from C2 in the plasma (and hence gas phase C2Hx species), relative to CH emission (which may be indicative of gas phase CHx species), led to poor quality deposits. We see poor quality deposits when most of the gas phase carbon is present as C2H2, and better quality deposits when most of the gas phase carbon is present as C1 species (CH4 and CH3).
The CH3 radical mole fraction detected can be quite sizeable (up to 10% of the total carbon introduced). It is well known that in the hot filament system, CH3 combination, followed by subsequent H atom abstraction reactions, is responsible for C2H2 generation [[25]]. Hence,
![]() Reaction
4.1.
Reaction
4.1.
For reaction 4.1 to be an important pathway in the plasma environment, H must be readily available in the gas phase to participate in the hydrogen abstraction reactions. Most of the source gas is H2, thus H is expected to be an abundant species, formed in the plasma by electron bombardment of H2 (reaction 4.2):
H2 + e- ® H + H + e- Reaction 4.2
Hence the overall reaction, 2 CH3 ® C2H2 (Reaction 4.1) is expected to be very rapid. If this were true, we would expect to find only low mole fractions of the intermediate species in reaction 4.1 (i.e. little C2H6 and C2H4). Further, for an overall rapid conversion of CH3 to C2H2:
![]() Eq. 4.1.
Eq. 4.1.
where K4.1 is an equilibrium constant.
In Fig. 4.10 the measured C2H2 mole fraction, [C2H2] has been plotted against the measured CH3 mole fraction squared, [CH3]2, for the data taken at the three levels of microwave power in Figs. 4.7-4.9.
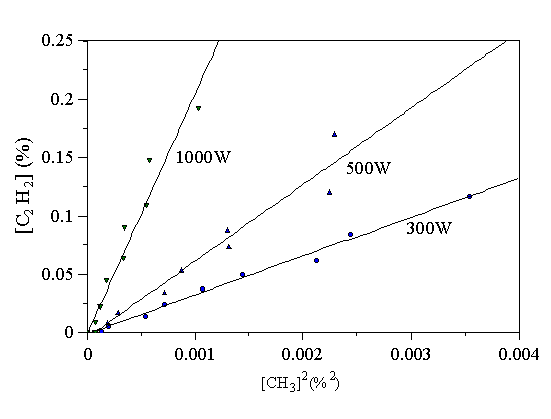
Figure 4.10 Graph showing measured [C2H2] against measured [CH3]2 for three levels of microwave power. Substrate temperature 700°C, 20 Torr pressure.
It is evident from Fig. 4.10 that Eq. 4.1 holds true at the three microwave power levels studied. From the gradients of the data in Fig. 4.10 it is possible to deduce that K4.1 = (8.7 ± 1)´105, (1.7 ± 0.5)´106, and (4.8 ± 0.5)´106 dm3 mol-1 at 300, 500, and 1000 W respectively (assuming ideal gas behaviour, with the gas temperature given by Fig. 5.1). Figs. 4.7-4.9 show low mole fractions of C2H4, and no C2H6 was detected under any experimental condition. These results are consistent with the view that H is an abundant species in the plasma, even at low powers, although its exact mole fraction is not known. Many early MWCVD experiments used low power microwave generators (~100 W), but still grew good quality diamond films [[26],[27]]. These results may be explained by the presence of H (inferred above), and hydrocarbon growth species (e.g. CH3, determined above), in significant quantities even at low microwave power levels.
The above experiments were repeated using C2H2/H2 and C2H4/H2 gas mixtures. Results were also taken using the power levels 300, 500, and 1000 W microwave power. Results for the C2H2/H2 gas mixtures are shown in Figs. 4.11-4.13, and results for C2H4/H2 gas mixtures are shown in Figs. 4.14-4.16.
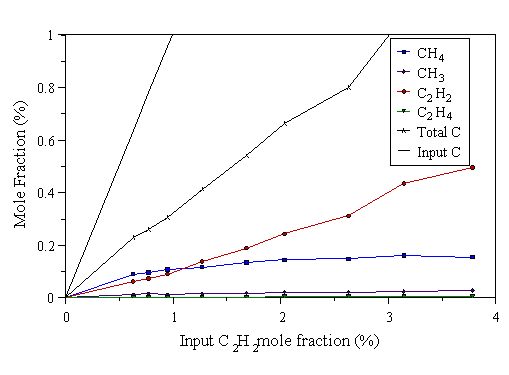
Figure 4.11 MBMS data taken from a 1000 W C2H2/H2 microwave plasma. 700°C substrate temperature, 20 Torr pressure.
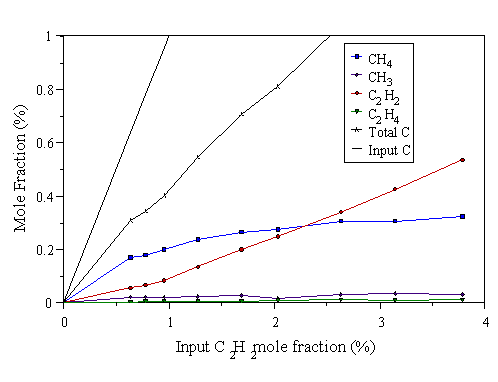
Figure 4.12 MBMS data taken from a 500 W C2H2/H2 microwave plasma. 700°C substrate temperature, 20 Torr pressure.
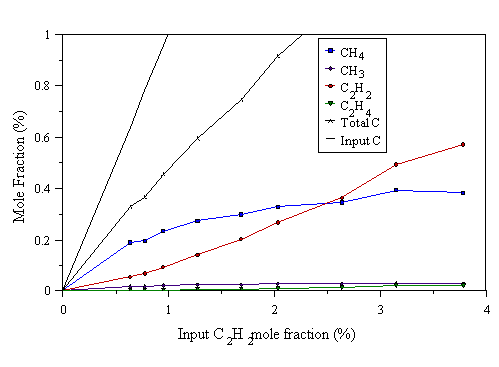
Figure 4.13 MBMS data taken from a 300 W C2H2/H2 microwave plasma. 700°C substrate temperature, 20 Torr pressure.
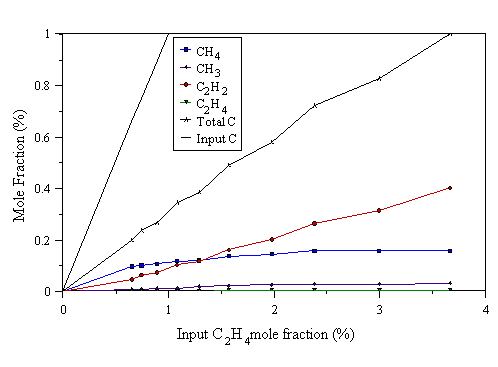
Figure 4.14 MBMS data taken from a 1000 W C2H4/H2 microwave plasma. 700°C substrate temperature, 20 Torr pressure.
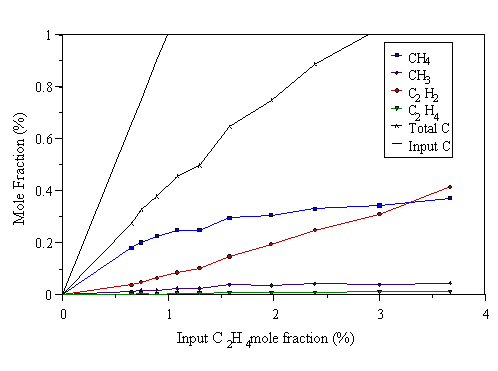
Figure 4.15 MBMS data taken from a 500 W C2H4/H2 microwave plasma. 700°C substrate temperature, 20 Torr pressure.
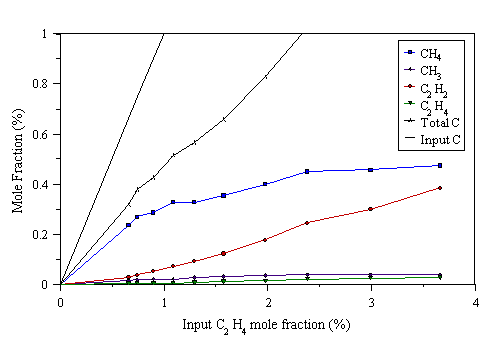
Figure 4.16 MBMS data taken from a 300 W C2H4/H2 microwave plasma. 700°C substrate temperature, 20 Torr pressure.
The previous six graphs show that at low input C2Hx mole fraction (x=2 or 4), reactions occur which break the C2Hx into C1 species, evidenced by the observed CH4, and the almost non-existent C2Hx detected. The chance of these C1 species subsequently meeting and reacting to form C2 species again is very small, as the input carbon level is low. Hence little C2Hx is observed. As the C2Hx mole fraction is increased, more C1 species are presumably formed by the break up of C2Hx. The increase of C1 species mole fractions in the gas phase increases the chance of C1 species meeting and reacting to form a C-C bond (i.e. C2Hx species), promoting the abundance of C2 species at high input C2Hx mole fraction.
For Figs. 4.11-4.16, Eq. 4.1 only holds for the microwave power level of 1000 W. This is because in the case of C2H2/H2, and C2H4/H2 mixtures, not all the input hydrocarbon has been dissociated at low powers. This is better demonstrated by the data in the next section (especially Figs. 4.21 and 4.22). Hence there is excess C2H2 in both cases which causes a plot of the kind in Fig. 4.10 to deviate from linearity at low powers.
4.5 Effect of
applied Microwave Power
After setting the CVD reactant gas composition, one of the next most important variables to be chosen is the microwave power used to sustain the discharge. This parameter (along with the reactor pressure) strongly affect the physical properties of the plasma, such as the electron density, electron energy distribution, and plasma volume [[28],[29]]. Using the microwave plasma system described in Chapter 3, it was possible to maintain a stable microwave plasma over the power range 200-1500 W with, or without the mass spectrometric diagnostic probe present. As 1500 W was the maximum rated output of the magnetron, a lower maximum working power of 1200 W was chosen for all work in this thesis.
4.5.1 Growth
Rates
Growth rates for diamond films grown with different microwave powers are presented in Fig. 4.17. The growth rate does not appear to be very dependent on the applied microwave power over the range studied here. The main effect of changing the microwave power over this range would seem to be to alter the size of the plasma ball. Growth rates obtained from films grown using the old substrate heater show much data point scatter, highlighting the reproducibility difficulties encountered in the presence of secondary plasmas.
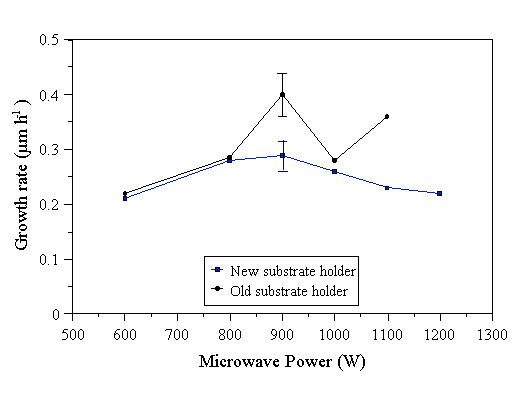
Figure 4.17 Growth rates of diamond films grown as a function of microwave power for a 2% CH4/H2 gas mixture. Constant conditions: 30 Torr pressure, 202 sccm total gas flow, 6 hours growth, 800°C substrate temperature (old substrate holder), 700°C substrate temperature (new substrate holder).
SEM images of diamond films grown with different levels of microwave power show no significant changes in morphology when using the old substrate holder (Figs. A.16-A.19). However, when using the new substrate holder, a marked variation in morphology was observed (Figs. A.20-A.24). At low powers (~600 W), a faceted film dominated by octahedral grains of typical size 0.5 mm was produced. As the power was increased to 800 W, large (1-1.5 mm), square (100) facets began to appear in the deposited film. At 900 W large, clean (100) facets comprised the most of the film surface. As the power was increased to 1000 W, the crystallite size decreased, twinning became more pronounced, and crystal facets became a mixture of (100) and (111). Above 1000 W, large, triangular (111) facets became the predominant morphological feature. The results obtained using the new substrate holder are consistent with the morphological changes seen when increasing the substrate temperature (see Section 4.8). The effect of increasing the microwave power is to make a larger plasma ball, which implies that a larger flux of hotter gas impinges on the substrate at higher power levels. This will not affect the substrate holder bulk temperature (the temperature controller ensures this remains at its set point). However, an increased flux of hotter gas will raise the local temperature of the deposition surface as the microwave power is increased. This local substrate temperature increase is the most likely explanation for the morphological variations reported above.
4.5.2 Film Quality
Representative Raman spectra of these films are given in Figs. A.16-A.24. The film quality, as judged by the Raman spectra, is also not greatly affected by the level of microwave power used for the deposition.
4.5.3 MBMS Gas
Phase Studies
MBMS measurements were taken as a function of microwave power. Again, it should be recalled that the plasma location relative to the sampling orifice is strongly dependent on the microwave power used (see Fig. 3.18). Results for a plasma in 2% CH4/H2 are shown overleaf in Fig. 4.18, and analogous graphs for 1% C2H2/H2 and 1% C2H4/H2 gas mixtures are presented in Figs. 4.19 and 4.20, respectively.
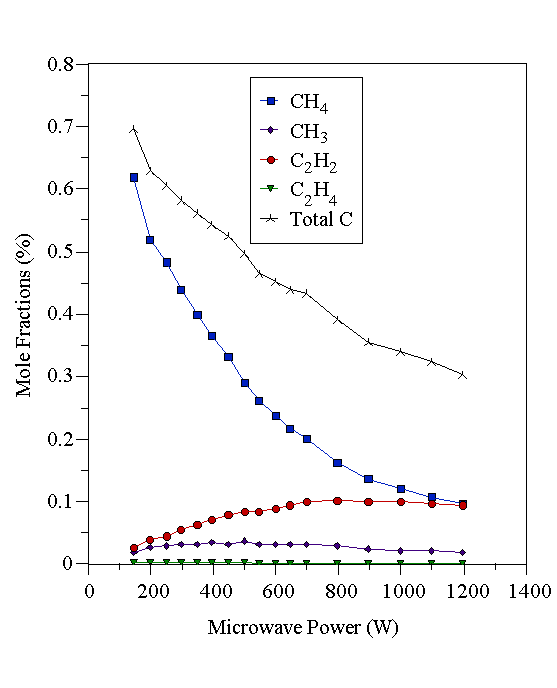
Figure 4.18 Plasma composition determined by MBMS using a 2% CH4/H2 feed gas mixture as a function of microwave power. Constant conditions: 700°C substrate temperature, 20 Torr pressure, 204 sccm total gas flow.
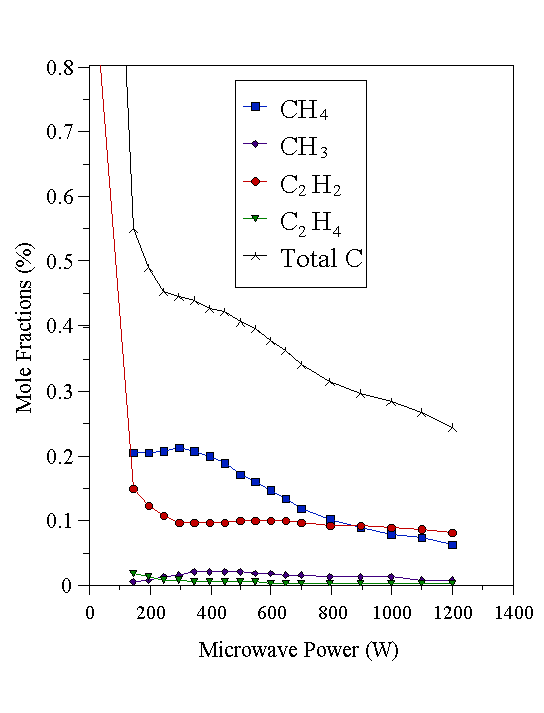
Figure 4.19 Plasma composition determined by MBMS using a 1% C2H2/H2 feed gas mixture as a function of microwave power. Constant conditions: 700°C substrate temperature, 20 Torr pressure, 202 sccm total gas flow.

Figure 4.20 Plasma composition determined by MBMS using a 1% C2H4/H2 feed gas mixture as a function of microwave power. Constant conditions: 700°C substrate temperature, 20 Torr pressure, 202 sccm total gas flow.
As the microwave power was increased, the total measured carbon was found to fall short of the input carbon, which (as explained in the previous section) we believe to be due to mass dependent thermal diffusion. This effect is plausible in a plasma system, as high powers (~1 kW) are deposited into a small region (~ a few cm diameter), where the electric component of the microwave field is strong enough to break down the gas [[30]]. This hot region is surrounded by cool gas, therefore a temperature gradient must exist between the hot and cool regions. Moreover, the effects of thermal diffusion have been observed experimentally many times in hot filament systems [[31]-[34]], so it is not surprising that they also exist in the plasma system. In the following chapter, evidence will be presented for a radial temperature gradient in the plasma which must contribute to the observed thermal diffusion.
At low powers (~200 W), carbon is present mainly in the form the input hydrocarbon reactant when using CH4/H2 and C2H2/H2 gas mixtures. In the case of 2% CH4/H2, CH3 and small quantities of C2H2 were also detected at low powers. As the power was increased, the amount of C2H2 increased, whilst the amount of CH4 decreased until approximately equal amounts were present at 1200 W, the highest power used. The CH3 mole fraction is remarkably constant compared with the variations seen for the CH4 and C2H2 mole fractions. CH3 is believed to be the dominant growth species in MWCVD [[35]], so these data complement the growth rate data (assuming CH3 as growth species), which were also quite insensitive to the level of microwave power. In the case of a C2H2/H2 gas mixture, there was formation of a significant quantity of CH4 at low powers, but at high powers the amounts of CH4 and C2H2 were approximately equal, as found for the 2% CH4/H2 mixture. The 1% C2H4/H2 source gas mixture was different again. At low powers the input C2H4 was almost totally destroyed, forming mainly CH4 and some C2H2. However, at high powers, the levels of CH4 and C2H2 produced became approximately equal, as was the case with the 2% CH4/H2 and 1% C2H2/H2 gas mixtures. Thus, at low powers the composition of the sampled gas (extracted from the region close to, but just inside, the visible plasma edge) was dependent on the nature of the hydrocarbon reactant. At high powers, a mixture containing similar amounts of CH4 and C2H2 was produced, irrespective of the nature of the hydrocarbon source. It is, however, difficult to separate the effects of the changing sampling position within the plasma from the effects that increasing the microwave power in isolation would have. What can be said is that at low powers the gas phase composition is dependent on the nature of the source gas, whilst at high powers a ‘soup’ of approximately uniform composition is produced regardless of hydrocarbon precursor. This can be illustrated by plotting the mole fractions of CH4 and C2H2 in Figs. 4.18-4.20 on separate graphs (Figs. 4.21 and 4.22 respectively). In these graphs, the CH4 and C2H2 mole fractions have been scaled up by a numerical factor to eliminate the microwave power dependence of thermal diffusion. This thermal diffusion correction makes the data easier to compare. The multiplying factor is calculated as the ratio of the input gas mixture C:H2 percentage to the measured total carbon percentage (as a function of microwave power). These data agree with the analysis of Bachmann et al [[36]], who reached the conclusion that a high power density supplied to the gas-phase, rather than a ‘magic’ CVD reactant composition was responsible for creating gas-phase species for diamond deposition.

Figure 4.21 Measured CH4 mole fractions as a function of microwave power, using three different source gas mixtures. Data adapted from Figs. 4.18-4.20 by correcting for the effects of thermal diffusion.
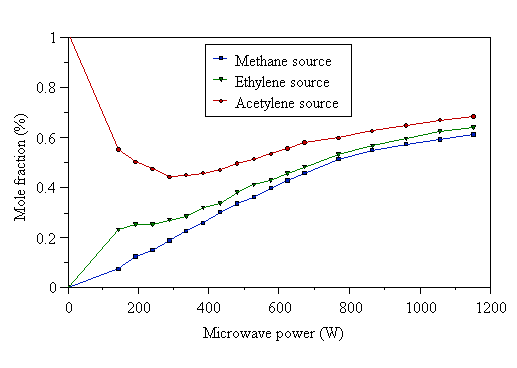
Figure 4.22 As Fig. 4.21, except measured C2H2 mole fractions are shown.
4.6 Effect of Reactor Pressure
It was possible to grow diamond films over the range 15-55 Torr using the MWCVD reactor described in Chapter 3. The size of the visible plasma region was strongly dependent on the reactor pressure, decreasing as the reactor pressure was increased. At 55 Torr the plasma was approximately 2 cm in diameter; any further increase in pressure caused the plasma to flicker visibly, and eventually jump from its central position.
4.6.1 Growth
Rates
Growth rate data as a function of pressure is shown in Fig. 4.23. Compared with other CVD reactor parameters (microwave power, substrate temperature), pressure has a more pronounced effect on the obtainable growth rates, the trend being for the growth rate to increase with CVD chamber pressure. As the CVD reactor pressure is increased, more reactants are available to reach the growing diamond surface, explaining the observed increase of growth rate with pressure. At low pressures, ~20 Torr, the entire 100 mm diameter substrate holder can be coated by diamond, however at higher pressures the diamond growth rate is reduced at the edges of the holder. This is evidenced by a grey circular patch ~40 mm diameter (corresponding to the region of diamond growth) observed after growth at ~50 Torr.

Figure 4.23 Growth rates as a function of CVD reactor pressure.
SEM images of the films grown with increasing reactor pressure are presented in Figs. A.25-A.29 (old substrate holder), and in Figs. A.30-A.32 (new substrate holder). There is no clear morphological trend evident for films grown using the old substrate holder, and again, this contrasts with the results for films grown using the new substrate holder. At 20 Torr, the film has many ‘clean’ facets, but no predominant texture. (111) facets become prevalent as the pressure is increased. The amount of twinning obtained also increased with pressure. (111) morphology is usually taken as indicating deposition at high substrate temperatures (as shown, for example, by the data in Section 4.8). This suggests that at higher pressures, a greater gas flux reaches the substrate, increasing the heat load that is transferred to the substrate, and thus raising the local temperature of the deposition surface. This local increase in substrate temperature results in the observed (111) morphology.
4.6.2 Film
Quality
Raman spectra of diamond films deposited over a range of CVD reactor pressures are also shown in Figs. A.25-A.32. These show that the diamond peak at 1332 cm-1 becomes more pronounced, relative to the other features of the spectrum, with increasing pressure, as observed in other studies of low pressure MWCVD diamond growth [[37]]. The diamond crystallite size was observed to increase with CVD chamber pressure (Fig. A.25-A.32), as with other MWCVD diamond growth studies [[38]].
4.6.3 MBMS Gas
Phase Studies
The MWCVD gas-phase composition was explored by MBMS, over the range 20-40 Torr in 2 Torr steps. This experiment required a slightly redesigned experimental procedure, adapted from that given in Section 3.6. Prior to the experiment backgrounds were taken in H2 for each species, at every pressure we planned to make mass spectrometric measurements under (i.e. 20, 22, 24,…, 38, 40 Torr). A fixed input C:H2 ratio of 2% was then set for the given hydrocarbon/H2 flow. A room temperature calibration measurement for that particular source hydrocarbon was made at each pressure to be studied (i.e. 20, 22,…, 38, 40 Torr). After these initial background and calibration measurements, the pressure was returned to 20 Torr. The plasma was struck, and the substrate heater controller set to provide a 700°C substrate temperature. Once the heater reached this condition, we took the first set of mass spectrometric readings at 600 W and 1000 W microwave power. These were checked to be steady over time, then the pressure was increased to 22 Torr, and the mass spectrometric measurements repeated, and so on until all pressures up to 40 Torr had been studied. The reactor was subsequently left to cool to room temperature, before being evacuated of gas. The remaining stable species to be calibrated were fed sequentially into the reactor and room temperature calibration measurements made at each pressure used in the experiment. Data were fed into a suitably modified AsEasyAs computer spreadsheet, and converted to mole fractions. Measured gas phase species mole fractions as a function of pressure are shown in Figs. 4.24 and 4.25 for 1% C2H2/H2, and Figs. 4.26 and 4.27 for a 1% C2H4/H2 gas mixture. The constant reactor conditions used for Figs. 4.24-4.27 were: 700°C substrate temperature (new substrate holder), and total gas flow 202 sccm. We were unable to gather meaningful data for a 2% CH4/H2 mixture, as these measurements were attempted whilst we had problems with the mass spectrometer signals rising over the period of the experiment. The data in Figs. 4.24-4.27 have not been corrected for the effect of thermal diffusion.
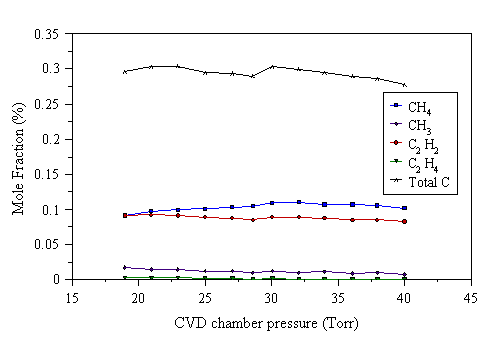
Figure 4.24 MBMS species mole fraction measurements as a function of pressure for a 1% C2H2/H2 reactant gas mixture under 1000 W applied power.
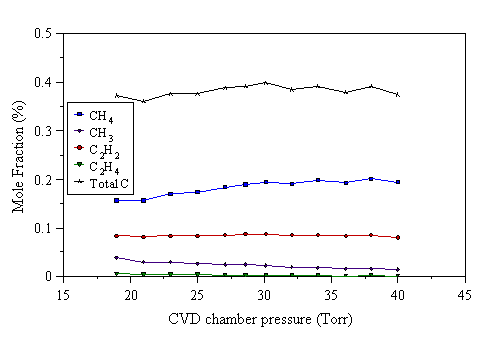
Figure 4.25 MBMS species mole fraction measurements as a function of pressure for a 1% C2H2/H2 reactant gas mixture under 600 W applied power.
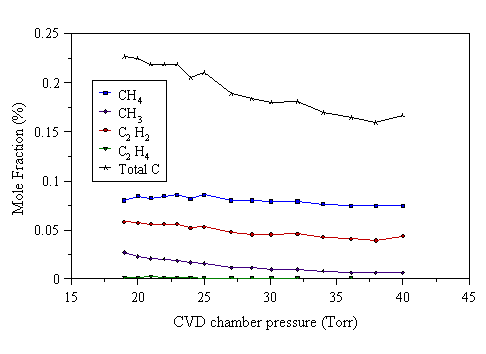
Figure 4.26 MBMS species mole fraction measurements as a function of pressure for a 1% C2H4/H2 reactant gas mixture under 1000 W applied power.

Figure 4.27 MBMS species mole fraction measurements as a function of pressure for a 1% C2H4/H2 reactant gas mixture under 600 W applied power.
Figs. 4.24-4.27 show that the mole fraction of C2H2 remains approximately constant with increasing reactor pressure. The mole fraction of CH3 drops as the pressure is increased, whilst that of CH4 increases. The formation of CH3 depends on atomic hydrogen, produced by electron impact dissociation, reacting with CH4. Atomic hydrogen can be destroyed in the gas phase by the third body process:
H + H + M ® H2 + M , Reaction 4.3
where M is another gas phase moiety.
At higher pressures, Reaction 4.3 will be more effective at removing H, due to the increased number densities of H and M. If a significant quantity of H is removed from the gas-phase, less CH3 will be formed, resulting in a fall of CH3 mole fraction with increasing pressure, as observed in Fig. 4.24-4.27.
4.7 Effect of Substrate (Vertical) Position
As mentioned in Section 3.2.3, one of the functions of the moveable substrate holder is to facilitate better ‘matching’ of the CVD chamber to the microwave circuit whilst attempting to strike the plasma. During this procedure, the substrate holder is moved vertically until the position with minimum reflected microwave power has been determined. Once the plasma has been struck, and the cavity re-tuned to account for the plasma load on the microwave circuit, the substrate holder can be translated through quite a large vertical distance whilst maintaining a stable plasma ball, and low reflected power. The effect of the substrate holder level in the reactor on diamond film growth, and gas phase composition at the (fixed) MBMS sampling point has been investigated.
Firstly, it was necessary to choose a co-ordinate system in the reactor to reference the measurements to. This vertical co-ordinate, z , is defined in Fig. 4.28, relative to the probe centreline (origin, O, on the reactor vertical axis) as this is fixed (the probe cannot move vertically). A radial co-ordinate, r, was also defined perpendicular to the reactor axis (z). This allows any point, P, in the reactor volume to be specified by the co-ordinate pair (z,r), e.g. the sampling position for probe 1 is at (0,15) mm, and at (0,25) mm for probe 2. Z refers to the substrate-sampling probe centreline separation.
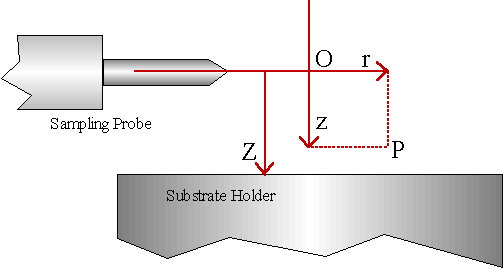
Figure 4.28 Definition of the co-ordinates z, r, and Z, used in experiments where the height of the substrate holder was varied.
As Z was varied, the plasma moved vertically relative to the substrate holder. Given that the plasma is a diffuse object with no solid edges to refer to, it is difficult to quantify this motion exactly. Typical motion of the plasma relative to the substrate holder is sketched in Fig. 4.29. The plasma becomes unstable as the substrate is lowered further than ~40 mm. Lengthening of the resonant cavity in such a manner alters the mode structure in the chamber, allowing the possibility of other modes existing simultaneously, and hence plasma instability.
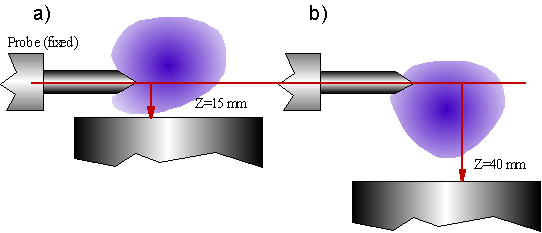
Figure 4.29 Sketch indicating the approximate plasma behaviour as the substrate holder is moved vertically.
4.7.1 Growth Rates
The growth rate of diamond films, deposited under identical conditions, as a function of Z is shown in Fig 4.30. It can be seen that the growth rate is approximately constant over the range of Z studied. The effect of vertical position on diamond growth has also been studied for a microwave plasma in a NIRIM-type reactor by Gonon et al [[39]], and by Pickrell et al [[40]]. Both groups found that operation of the NIRIM-type reactor in a ‘remote’ configuration (i.e. plasma not in contact with substrate) reduced the amount of secondary nucleation, and the amount of non-diamond phases incorporated into the film, compared with the traditional NIRIM mode of operation, where the substrate is immersed in the plasma.
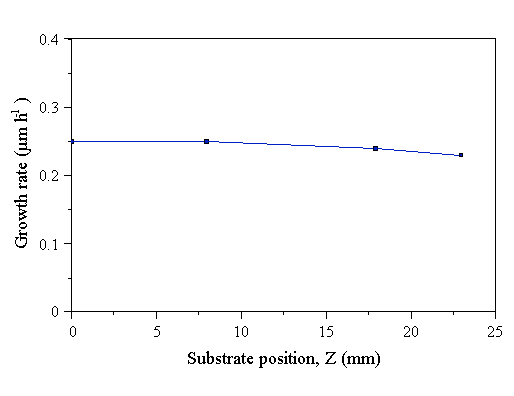
Figure 4.30 Growth rate of CVD diamond films as a function of the vertical position of the substrate in the reactor. Constant experimental conditions: 1000 W microwave power, 700°C substrate temperature, and 20 Torr pressure, 0.72% CH4/H2. Sampling probe not present.
The growth rate does not depend strongly on the vertical position of the substrate holder. This is perhaps because, as the substrate position is lowered (increasing Z), the plasma only moves a small amount upwards relative to the substrate holder. The flux of species incident at the substrate surface is dominated by diffusion in low pressure MWCVD systems [25], thus the gas phase species responsible for diamond growth, formed in the plasma, are still able to reach the substrate at similar fluxes for all vertical positions of the substrate attainable in the reactor. In addition, the film deposited at Z=23 mm (Fig. A.33) has the same morphology as that deposited at Z=0 mm (Fig. A.12), indicating that the small change in plasma location induced by varying Z does not affect the temperature of the deposition surface significantly.
4.7.2 MBMS Gas Phase Studies
We have undertaken MBMS measurements of the gas phase composition as a function of Z. The effect of the substrate holder position, using a 1% CH4/H2 reactant gas mixture, on the gas phase composition is presented in Fig. 4.31. The purpose of this experiment is to produce a vertical spatial species mole fraction profile through the plasma.
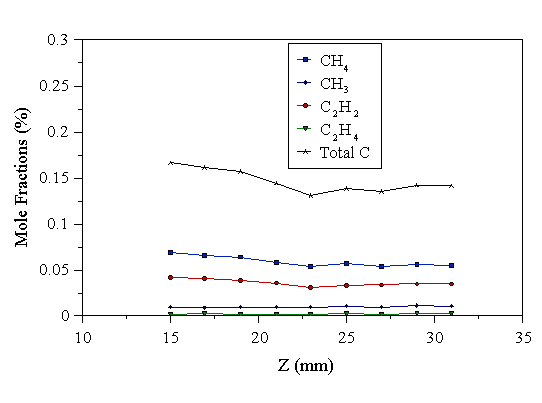
Figure 4.31 MBMS measurements of the gas phase composition Z mm above the substrate holder. Constant experimental conditions: 1000 W microwave power, 700°C substrate temperature, and 20 Torr pressure, 1% CH4/H2 input.
From these data, it can be concluded that (within this narrow range) the vertical position of the substrate in the reactor does not significantly affect the deposition rate (or quality) of the diamond films, or the gas phase composition at the sampling point. It should be borne in mind that as the substrate holder is moved vertically the plasma follows it, so the sampling location is also changing vertically relative to the plasma. Therefore; it appears that the plasma does not change its composition much over the ~30 mm of plasma height studied here. In Chapter 5, it has been shown that the temperature profile through the plasma is also almost completely insensitive to the vertical position of the plasma relative to the sampling probe, over the vertical range studied here.
4.8 Effect of Substrate Temperature
As mentioned in Section 1.3, diamond growth occurs in an optimum substrate temperature range 600-1100 K [[41]]. We have investigated the effect of the substrate temperature on the growth of diamond films, and on the gas phase composition above the heated substrate. Recall that our substrate temperature is that measured by the thermocouple in the substrate holder, and as such is probably not the same as the true substrate temperature.
4.8.1 Growth Rates
The growth rate of diamond films as a function of substrate (i.e. thermocouple) temperature is shown below in Fig. 4.32. The lowest temperature point corresponds to the case where no additional substrate heating was supplied - substrate heating occurred only by heating due to the plasma gas. Even in this case, CVD diamond could be grown, although at a lower rate than was possible with substrate heating. Again, the films grown using the old substrate holder, in the presence of secondary plasmas, show considerable scatter in growth rates.
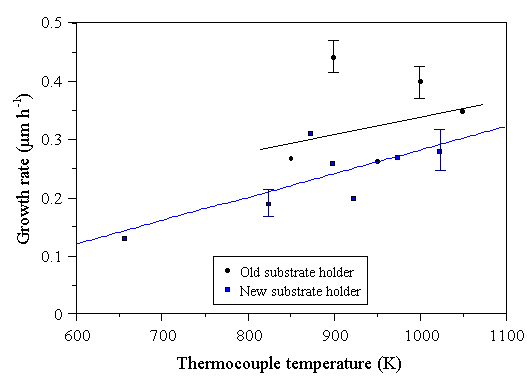
Figure 4.32 Growth rate of MWCVD diamond films as a function of substrate temperature. Constant conditions: 1000 W microwave power, 30 Torr pressure, 0.72% CH4/H2 for 6 hours. 202 sccm total gas flow.
4.8.2 Film Quality
SEM and Raman data for these films can be found in Figs. A.34-A.37, and Figs. A.38-A.43, for the old and new substrate holders, respectively. Using the old substrate holder, small grains with few well defined facets are again observed. As the substrate temperature increases, facets become larger and better defined. However, when diamond films grown using the new substrate holder are examined, more striking morphological trends are observed. At low substrate temperatures (plasma gas heating only), the crystals are well defined, being predominantly octahedral. As the substrate temperature increases, these become truncated octahedra. At ~870 K, large, smooth, square (100) faces predominate. At 920 K however, the morphology has again shifted to twinned, triangular (111) faceting. At 970 K the film still retains (111) faceting, but exhibits more twinning than the films deposited at lower temperatures.
4.8.3 MBMS Gas Phase Studies
MBMS measurements were taken as a function of substrate temperature, and are shown in Fig. 4.33. The substrate-sampling point distance was kept constant at Z=20 mm. The mole fractions of CH4, C2H2, C2H4, and CH3 were studied by MBMS, as the level of substrate heating was increased.
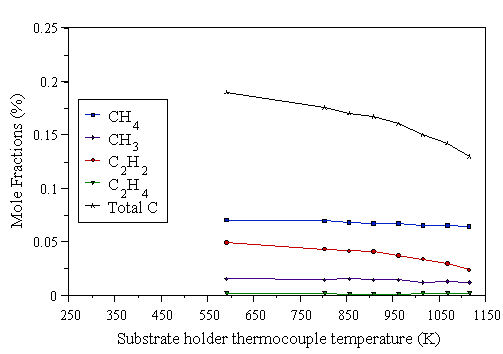
Figure 4.33 Dependence of gas phase species mole fractions, at 20 mm above the substrate, as a function of substrate temperature. Conditions: 1000 W microwave power, 20 Torr pressure, 1% CH4/H2 reactants, 202 sccm total gas flow.
Once again, we see the effects of mass dependent thermal diffusion in the MBMS data. As the substrate is heated, gas above it is also heated. This produces steeper temperature gradients, and hence enhanced thermal diffusion, resulting in the drop of the total measured carbon. Below a substrate temperature of 750 K, substrate heating has little effect on the measured species mole fractions. Presumably, under these conditions the substrate temperature is too low to initiate significant gas-surface chemistry, and hence the gas composition above the substrate remains unperturbed by the presence of the unreactive substrate. Above 750 K, the measured species mole fractions begin to show a dependence on the substrate temperature, presumably indicating that the substrate has reached the temperature regime where gas-surface chemical effects come into play. It is interesting to note that the C2H2 mole fraction falls (relative to the carbon balance), and the CH4 and CH3 mole fractions rise (relative to the carbon balance), as the substrate temperature is increased. It has been speculated that as there is no rapid gas-phase reaction pathway for C2®C1 species conversion, production of C1 hydrocarbon species (when using a C2Hx species as the carbon source gas) arises from C2Hx species thermal dissociation on the heated substrate surface [25,[42]]. This would presumably be more efficient at higher substrate temperatures, in which case the substrate would be seen to act as a sink for C2 species. Such dissociation of C2 species at the substrate surface would lead to free C1 gas-phase species. The diamond surface is almost entirely hydrogen terminated under CVD diamond growth conditions, and hence most C1 species (produced by C2 species dissociation) would not add to the diamond lattice, but instead return to the gas-phase. This would affect the gaseous composition in the proximity of the substrate by enriching the C1 gas-phase species at the expense of C2Hx species, as observed in the data of Fig. 4.33.
4.9 Effect of Probe Length - Probe 2 MBMS
measurements
As described in Section 3.3.1, probe 2 was fabricated to be 10 mm shorter than probe 1. Probe 2 was used to attempt some of the mass spectrometric experiments described in Section 4.5.3. Being shorter, probe 2 does not penetrate the plasma to the same extent as probe 1. Indeed, the tip of probe 2 does not glow when it touches the ‘normal’ plasma region indicating that the sampled gas is significantly cooler in the vicinity of the sampling region of probe 2. The experiment described in Section 4.5.3 was repeated, where the effect of applied microwave power was examined. These results are presented in Fig. 4.34.
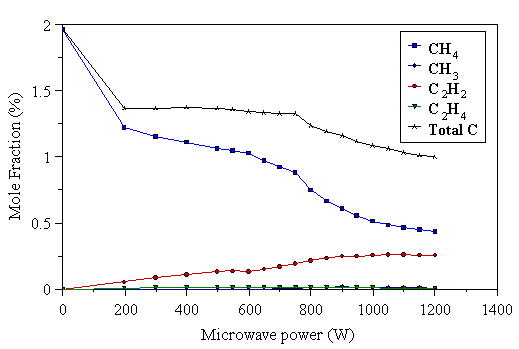
Figure 4.34 Gas phase composition determined using probe 2 (sampling point at r=25 mm from the reactor axis). 2% CH4/H2 gas mixture, 700°C substrate temperature, 20 Torr pressure, 204 sccm total gas flow.
At high powers, these results show similar qualitative behaviour to those obtained with probe 1 (Fig. 4.18), however there are differences. This experiment was performed starting at high applied powers, gradually lowering the power in steps. When the power had been reduced to about 700 W, the plasma had contracted enough that it was no longer in contact with the probe. The plasma was also noted to be less luminous than with probe 1 below 900 W. As the microwave power level was reduced further still, the plasma became smaller and less luminous before jumping into the gap at the side of the substrate holder when the power reached ~600 W. The measurements below 600 W were taken with the plasma positioned at the side of the substrate holder. The total measured carbon at these powers is much closer to that input, compared with the results for probe 1, indicating little thermal diffusion at the sampling point. This is consistent with the plasma being ~5 cm from the sampling orifice, at the side of the substrate holder, and thus the temperature gradient in the sampling region is significantly reduced. The sampled gas is only heated by the substrate heater, and largely has the input gas composition, however there are differences caused by species produced in the plasma diffusing into the sampling region (e.g. the presence of C2H2). The CH3 mole fractions measured with probe 2 (25 mm from the plasma centre) are lower than those measured with probe 1 (15 mm from the plasma centre), and highlight the reactive nature of this species.
4.10 References