Chapter 6 -Results for C/H/N Systems
“I don’t know what it was, but it had either prussic acid or white lead in it. I should fancy it was prussic acid, as she seems to have died instantaneously.”
Oscar Wilde ‘The Picture of Dorian Gray’
6.1 Introduction
Nitrogen is the most common impurity found in natural diamond [[1]]. Natural diamond is a good electrical insulator, however, when the doping level (the amount of impurity atoms, e.g. nitrogen, incorporated into the diamond lattice) increases, the electrical properties of the diamond change to those of a semiconductor. The potential of diamond to be both insulator and semiconductor has led many people to conceive of novel diamond-based micro-electronic devices. Much interest has also been expressed in making so-called ‘cold cathode’ electron emitters [[2]-[4]], and field emission displays from doped diamond [[5]].
The modern era of CVD diamond growth allows diamond to be deposited as a thin film, on Si wafers (as used in the micro-electronics industry). In order to make diamond semiconducting, it is necessary to introduce a second element (the dopant), with a different electronic structure to carbon, into the diamond lattice. Attention has mainly focused on group 13 or group 15 elements (such as boron [[6]-[8]], nitrogen [[9]-[11]], or phosphorus [[12]-[14]]) as possible dopants for CVD diamond. This may be done by post-deposition ion implantation [13,[15],[16]], but a more attractive approach would be to carry out the doping during growth by the addition of a dopant gas to the CVD process gas mixture (typically CH4/H2).
The presence of nitrogen in the CVD reactant gases is known to affect the film morphology, quality, and growth rate when producing diamond films by MWCVD [9,[17],[18]]. Nitrogen (as N2) added to a typical CH4/H2 CVD mixture in the 10-100 ppm (parts per million) range was found to promote the formation of (100) surface texture, and produce a ~ fivefold increase in growth rate over that obtained from CH4/H2 alone [9,[19],[20]]. At higher levels of nitrogen input the growth rate [10,19,[21]] and film quality [19,21] (assessed by Raman spectroscopy), was found to decrease. In addition to examining surface morphology, many previous studies of nitrogen addition to diamond CVD reactants have focused on the incorporation of nitrogen into the growing film [[22],[23]]. This was found to be quite inefficient, in that only a small amount of the input nitrogen (relative to the input carbon) was incorporated into the film. There is, to date, no firm consensus as to whether gas-surface, or gas-phase chemical reactions (or a combination of both) are necessary to account for these observations. Most studies have concentrated on the properties of films deposited in the presence of nitrogen, and hence neglected the fate of nitrogen in the gas-phase, prior to incorporation into the growing film. In order to supplement the data concerning properties of diamond films deposited in the presence of nitrogen, this chapter presents a study of the gas-phase composition during MWCVD with nitrogen present in the CVD feed gas. The technique of in situ molecular beam mass spectrometry (MBMS) [[24],[25]] has been used to determine concentrations of stable and radical species in the plasma, and the results have been published in Diamond & Related Materials [[26]]. This study parallels previous work carried out in the Bristol laboratories for hot filament CVD by Tsang et al [[27]]. Some growth experiments have also been carried out, and the amount of nitrogen incorporated into the film has been examined using SIMS (Chapter 2), and the results published in Thin Solid Films [[28]].
6.2 Effect of applied Microwave Power
A carbon to nitrogen ratio, C:N=1:1 was used in this study. Nitrogen was added to a typical 1% C:H2 CVD gas mixture in one of the following forms: N2, NH3, CH3NH2, or HCN. For the experiments using nitrogen as N2 and NH3, CH4 was used as the carbon source. CH3NH2 and HCN required no extra carbon to be added to the system. These four nitrogen-containing gases were chosen as the nitrogen occurs in a variety of chemically bonded forms (i.e. NºN, N-H, C-N, and CºN). The effect of microwave power on the gas phase composition was investigated initially, as this parameter seemed to have a marked effect on the gas-phase composition measured by MBMS, when hydrocarbon/H2 source gas mixtures were used (Chapter 4).
The same techniques were used for the MBMS determination of gas-phase composition as those of Tsang et al [27,[29]], who performed an analogous study of the effect of nitrogen additions during HFCVD diamond growth. There were, however, some slight differences in methodology between that work and the present study. Chiefly, we used the modified data correction procedure given in Section 3.8.5. As noted in that section, this was to ensure that the background corrected data did not become negative. Additionally, electron ioniser energies had to be chosen for the detection of the nitrogen containing species. These are given in Table 6.1.
|
m/q |
Species
detected |
Electron
energy used for detection (eV) |
|
2 |
H2 |
16.0 |
|
15 |
CH3 |
13.6 |
|
16 |
CH4 |
16.0 |
|
17 |
NH3 |
13.6 |
|
26 |
C2H2 |
13.2 |
|
27 |
HCN |
16.0 |
|
28 |
C2H4 |
11.9 |
|
28 |
N2 |
30.0 |
|
31 |
CH3NH2 |
13.6 |
Table 6.1 Electron energies used to detect species for all experiments in this chapter.
One complication arises from the fact that C2H4 and N2 have equal masses (28 a.m.u.), hence the mass spectrometer measures a combined C2H4+N2 signal, I(C2H4+N2), at 30.0 eV. In order to determine I(N2) separately, an additional correction to those described in Chapter 3 must be carried out to subtract the C2H4 signal from the combined signal, I(C2H4+N2). At 11.9 eV C2H4 can be detected (as IP(C2H4) = 10.51 eV), but N2 cannot (as IP(N2) = 15.55 eV). Therefore the 11.9 eV m/q=28 signal arises solely from the species C2H4. During stable species calibration, we measure signals for a known concentration (e.g. 1%) of C2H4 at 11.9 eV (as in, for example, Chapter 4) but also at 30.0 eV (the energy at which the combined signal, I(C2H4+N2), is measured). Therefore, the C2H4 signal at 11.9 eV can be related to the C2H4 signal at 30.0 eV, and a subtraction made to correct the 30.0 eV I(C2H4+N2) data for the C2H4 contribution, yielding I(N2).
This procedure was found to be satisfactory, however the data produced by this process sometimes produced highly scattered data, as noticed during the previous HFCVD studies [29,[30]]. N2 presents several experimental detection problems, as it is present in all stages of the vacuum system as a consequence of the non-zero base pressure attainable by the vacuum pumps. Combined with the interference of C2H4 at the same m/q, and the extra corrective procedure this implies, it is not surprising that the N2 signal should be harder to determine accurately. For most of the experiments this was not too much of a problem. In one case, however (Fig. 6.1), where there was a significant quantity of N2 present, it was necessary to use another approach to determine the N2 mole fraction. A C:N feed ratio of 1:1 was used in most experiments (Section 6.2.1-6.2.4). It was therefore possible to require the total measured N mole fraction to equal the total measured C mole fraction, in order to be consistent with the input values. As all the other nitrogen containing species of interest had their mole fractions determined in the MBMS experiment, an upper bound to the N2 mole fraction could be estimated by subtracting the mole fraction of all measured N-containing species from the total N mole fraction ( = total C mole fraction), and halving the result (N2 is diatomic). It was only found to be necessary to do this for one particular experiment (Fig. 6.1).
6.2.1 1% CH4 / 0.5% N2 / H2
Gas Mixture
The gas-phase composition determined by MBMS for a 1% CH4/0.5% N2/H2 gas mixture at 20 Torr is shown as a function of microwave power in Fig. 6.1 (overleaf). These data should be compared with the analogous graph in Chapter 4 for a CH4/H2 gas mixture (Fig. 4.18). As in that case, the measured carbon total decreased as the microwave power was increased, due to mass-dependent thermal diffusion in the plasma. The CH4 mole fraction fell, and C2H2 was produced, as in the case of hydrocarbon/H2 feed gases (Chapter 4). However, some HCN (containing ~5% maximum of the input N), and NH3, are also produced, indicating that a small proportion of the input N2, with its strong NºN bond, is dissociated in the plasma.
Table 6.1 Key to all figures in this Chapter:
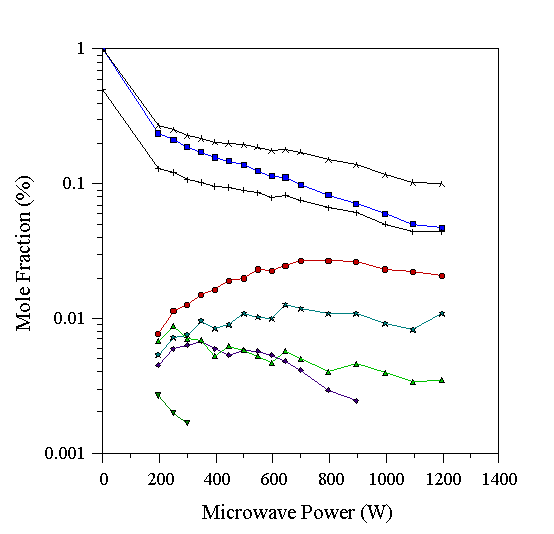
Figure 6.1. Graph showing species mole fractions determined by MBMS for a 1% CH4/0.5% N2/H2 microwave plasma. Conditions 20 Torr, 700°C substrate temperature. See Table 6.1 for key to symbols.
The comparative unreactivity of N2 under these conditions allows the ‘normal’ gas-phase reactions encountered in diamond CVD using CH4/H2 gas mixtures to proceed almost unimpeded. This is evidenced by the production of C2H2 at high microwave powers, as in the results of Chapter 4. These results are also similar to those seen during HFCVD at low filament temperatures (~1600°C), where very little N2 dissociation was found to occur [27,29]. At high filament temperatures more N2 was found to dissociate, which was not seen under the conditions of this study. This indicates that the hot filament dissociates N2 more readily than the plasma environment (under the conditions of this study). In the hot filament system gas activation is believed to occur on the filament surface (~2600 K), whereas in MWCVD, activation occurs throughout the plasma whose temperature is typically 1400 K [[31]], also reported in Chapter 5. Thus the results for MWCVD can be compared meaningfully with those for HFCVD when the temperature of the activation zone (filament/plasma) is similar. It may be possible to decompose a larger fraction of the input N2 in the microwave plasma by using higher pressures, or higher microwave powers, due to the increased gas temperature under these conditions.
6.2.2 1% CH4 / 1% NH3 / H2 Gas Mixture
Fig. 6.2 shows the effect of using a 1% CH4/1% NH3 mixture in H2. As the microwave power was increased, NH3 and CH4 were consumed in the plasma, presumably by reaction with H to produce NH2 and (the observed) CH3. NH2 radicals, and/or CH3 radicals are necessary for the production of species containing a C-N bond (Reactions 6.1 and 6.2). H abstraction reactions of such a species containing a C-N bond will result in the formation of the highly stable moiety HCN.
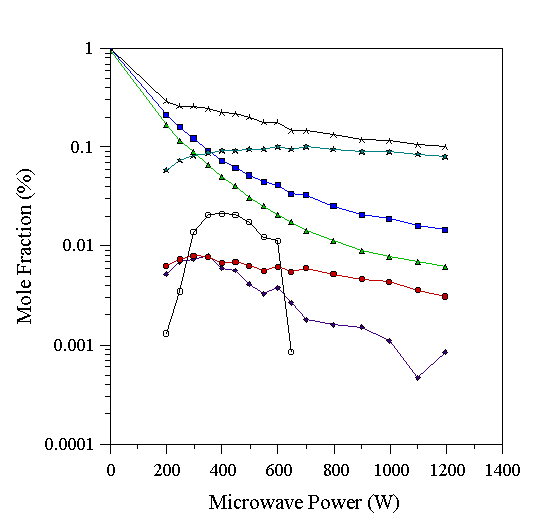
Figure 6.2. Graph showing species mole fractions determined by MBMS for a 1% CH4/1% NH3/H2 microwave plasma. Conditions 20 Torr, 700°C substrate temperature. See Table 6.1 for key to symbols.
Fig. 6.2 shows that the NH3 mole fraction falls more dramatically with microwave power than the CH4 mole fraction. This observation was also noted in the MBMS study of Tsang [29], where the NH3 mole fraction fell more rapidly than the CH4 mole fraction as a function of filament temperature. It was concluded that this indicated a higher NH2 mole fraction than CH3 mole fraction. Further, Tsang concluded that this made the reaction
CH4 + ·NH2 ® CH3NH2 + H·, Reaction 6.1
more likely than the reaction
·CH3 + NH3 ® CH3NH2 + H·, Reaction 6.2
for the formation of C-N bonded species, due to the greater abundance of NH2 and CH4, compared to the abundances of NH3 and CH3. The · symbol is used here to emphasise the radical species in Reactions 6.1 and 6.2. The present results indicate that the mechanism for C-N bond formation (Reaction 6.1) may also be important in the microwave plasma environment.
Between 200 W and 600 W a sizeable signal at m/q=28 was detected in this experiment (and in those reported in sections 6.2.3 and 6.2.4). Common ions with m/q=28 are CO+, N2+, and C2H4+. Out of these species, only C2H4 could ionise at the electron kinetic energy used, but a high C2H4 concentration would be very surprising given the previous work on hydrocarbon/H2 mixtures, which showed C2H4 to be present at a concentration close to the ultimate resolution of our instrument under all conditions studied (Chapter 4, and [31]). Hence the m/q=28 signal is not believed to be due to C2H4. Instead, Fig. 6.2 shows that by increasing the applied power, the conversion of CH4 and NH3 to HCN is being effected. This requires an intermediate species containing a C-N bond, which will undergo H abstraction reactions to form HCN. Species such as CH3NH2, CH2NH2, or CH3NH are possible intermediates. The latter two are radical species, and will have low ionisation potentials. Electron bombardment of CH2NH2 would produce CH2=NH2+ and, conceivably, HCºNH+ by dissociative ionisation. HCºNH+ has m/q=28, and a stable electronic structure analogous to C2H2. Consistent with this view, the m/q=28 signal is found at power levels where the NH3 and CH4 signals are falling, yet where the HCN mole fraction is rising, as would be expected for an intermediate in the conversion of CH4 and NH3 to HCN. We thus assign the m/q=28 signal to a radical species, which we cannot calibrate to produce an absolute mole fraction. Therefore measured carbon totals on all graphs in this chapter are those determined excluding the m/q=28 signal. The m/q=28 signal is thus in arbitrary units (a.u.) in Figs. 6.1-6.4.
6.2.3 1% CH3NH2 / H2 Gas Mixture
The gas-phase composition determined using CH3NH2 as the source of C and N is shown in Fig. 6.3. Even at the lowest powers used, the CH3NH2 concentration falls close to the minimum detectable, indicating rapid destruction of CH3NH2 in the plasma environment. CH4, NH3 and HCN are observed instead. Two reaction pathways arise for a CH3NH2 molecule in the plasma. Firstly, H abstraction reactions could occur, producing a strong, stable CºN bond (as in the observed product HCN). This is analogous to the chemistry observed in hydrocarbon/H2 gas mixtures, where the CH3 combination reaction (Reaction 4.1), followed by H abstraction reactions produced C2H2. Secondly, fission of the C-N bond in the CH3NH2 molecule is possible, followed by reaction with H or H2 to produce the observed products CH4 and NH3. The measured CH4 and NH3 concentrations track each other closely, as would be expected if they were produced by fission of the C-N bond in CH3NH2. The amounts of CH4 and NH3 are reduced with increasing microwave power, with a concomitant rise in the HCN mole fraction, relative to the total measured carbon mole fraction. Again, a sizeable quantity of species with m/q=28 was measured in the low power region (200-600 W), where the conversion of CH4 and NH3 to HCN occurs.
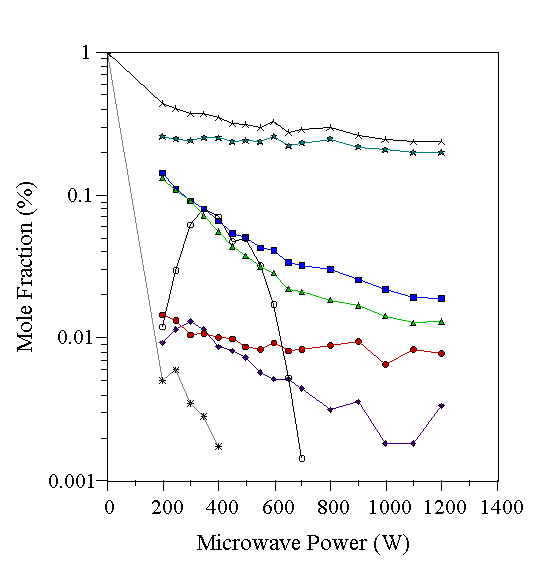
Figure 6.3 Graph showing species mole fractions determined by MBMS for a 1% CH3NH2/H2 microwave plasma. Conditions 20 Torr, 700°C substrate temperature. See Table 6.1 for key to symbols.
6.2.4 0.5% HCN / H2 Gas Mixture
As mentioned earlier, only a small quantity of HCN was synthesised for each experiment, and therefore less MBMS data were taken for this gas. A smaller C:N:H2=1:1:200 was also used for this experiment to make the HCN supply last longer. Approximately 0.5 dm3 HCN was produced by reaction of 1 g NaCN (0.02 mol) with excess 0.2 M H3PO4, in vacuo. A diagram of the apparatus used for this synthesis is shown in Figure 6.4. The gas was produced in flask #1, and collected in flask #2, using liquid N2 to freeze the HCN in the ‘cold finger’.
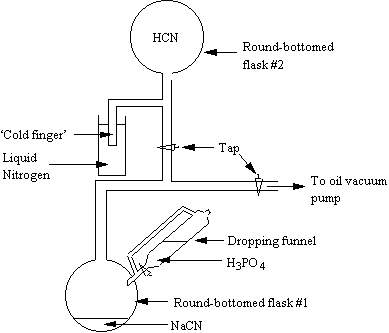
Figure 6.4 Vacuum line apparatus used in the synthesis of HCN for the experiment described in Section 6.2.4.
The gas-phase composition for a 0.5% HCN/H2 plasma, determined by MBMS is shown in Fig. 6.5. Most of the gas remains as HCN, but at low powers small quantities of CH4 and NH3 are seen. The fact that little HCN is decomposed emphasises the stability of the HCN molecule under conditions found in the type of plasma discharge studied here. The situation for HCN is very similar to that of N2 noted previously - both are highly stable in the plasma conditions used here. Once again, below ~600 W, a significant m/q=28 signal was observed.
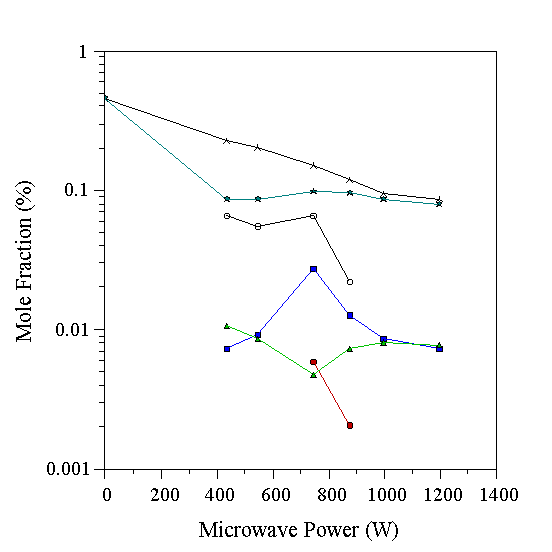
Figure 6.5 Graph showing species mole fractions determined by MBMS for a 0.5% HCN/H2 microwave plasma. Conditions 20 Torr, 700°C substrate temperature. See Table 6.1 for key to symbols.
6.3.1 Effect of Nitrogen in the gas-phase using N2
as Nitrogen source
Diamond films were deposited from a 1% CH4/H2 gas mixture, with N2 added in the range 0-4.7%. This produced smooth nanocrystalline films ~1 mm thick after 6 hours deposition for all experiments where N2 was added (minimum N2 added was 0.5%), and a faceted diamond film when no N2 was added. The variation of growth rate with input N2 mole fraction is presented in Fig. 6.6.
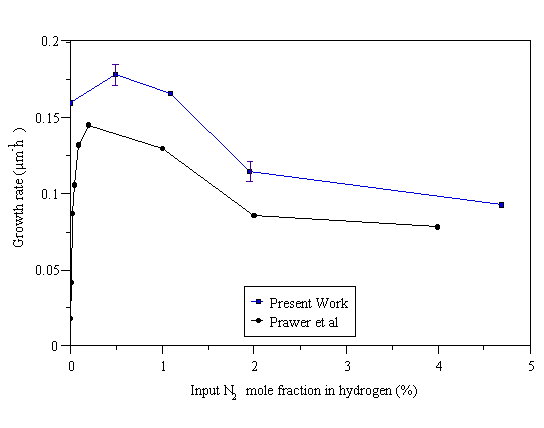
Figure 6.6 Variation of diamond film growth rate as a function of N2 mole fraction added to a 1% CH4/H2 feed. Conditions: 1000 W microwave power, 700°C substrate temperature, 20 Torr pressure. Also shown are the results of Prawer et al [19], which have been divided by a factor of 10 for comparison on the same graph.
Also shown are the results of Prawer et al [19], who were able to examine the region where very little N2 was added (<1000 ppm), which could not be investigated in the present study due to the comparatively large 0-10 sccm MFC employed to deliver the N2. Also, the present growth rate ‘without’ nitrogen in the feed is very high compared with the results of Prawer et al. This is consistent with the view that a small quantity of N2 was in fact present in the CVD chamber as a consequence of the non-zero base pressure attainable by the vacuum pumps, small leaks in the vacuum system, or as an impurity in the gas supplies.
The diamond films examined in Fig. 6.6, grown using a 1% CH4 gas mixture with N2 mole fractions of 0-4.7%, were examined for incorporated nitrogen by the technique of secondary ion mass spectrometry (SIMS) [28]. In common with previous authors it was found that nitrogen has a very low incorporation efficiency (<10-3) [22,23], even when several percent of the CVD feed gas is N2.
A 1% CH4/H2 plasma was examined by MBMS as a function of the mole fraction of N2 added to the feed, and the results are shown in Fig. 6.7. The measured carbon total remains constant at a lower value than the input amount due to thermal diffusion effects. As the mole fraction of N2 was increased, the mole fractions of the hydrocarbon species were reduced slightly, due to the formation of HCN. This indicates that under these conditions N2 only participates in the gas phase chemistry to a limited extent, even when several percent of N2 is added. This has implications for growth studies using N2 additions to form p-type diamond [9,10,17-23], since almost all added nitrogen remains as gas phase N2 (under the conditions of this study).
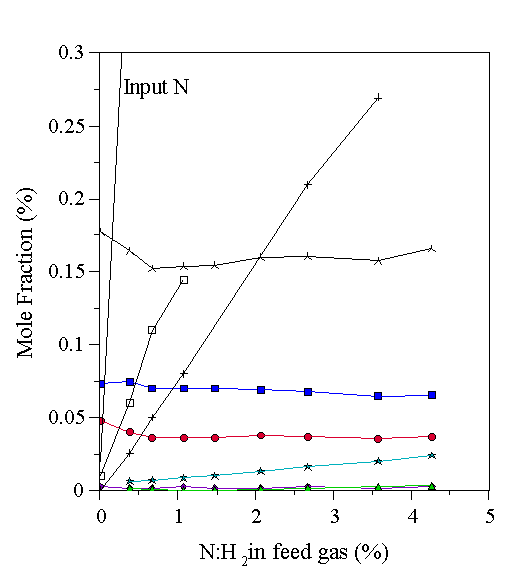
Figure 6.7 Gas-phase composition of a 1% CH4/H2 microwave plasma as a function of added N2, determined by MBMS. See Table 6.1 for key to symbols.
6.3.2 Effect of Nitrogen in the gas phase using NH3
as Nitrogen source
The previous MBMS experiment was repeated with the addition of NH3 to the feed gas instead of N2. The data are shown in Fig. 6.8 and it is apparent that the NH3 reacts more readily than N2 with the hydrocarbon species to produce HCN. Most of the measured carbon has been ‘locked up’ as HCN by the time the NH3 concentration becomes stoichiometric with the input CH4 (1%). Once the NH3 concentration increases beyond the 1% input carbon limit additional HCN can no longer be formed, and excess NH3 is largely converted to N2. Presumably this occurs in an analogous fashion to the case of CH3 combination followed by H abstraction reactions forming C2H2, which occurs in the absence of nitrogen. For nitrogen, however, NHx species (x=0-3) can combine to produce N2Hy (y=0-4). Such species were not observed using the molecular beam mass spectrometer, indicating rapid destruction in the plasma environment, presumably by H atom abstraction reactions to produce the observed N2. These results suggest a method to selectively tailor the gas phase composition between species containing one nitrogen atom and N2 by varying the concentration of ammonia relative to CH4.

Figure 6.8 Gas-phase composition of a 1% CH4/H2 microwave plasma as a function of added NH3, determined by MBMS. See Table 6.1 for key to symbols.
6.4 References