Adsorption of Polyelectrolytes at the Clay/Water Interface

Andy Nelson (previous student, left the group in 2003)
Kaolinite has many uses in industry; the majority is used in paper manufacture as a pigment or filler. Other uses include porcelain, extenders in paints, etc. The kaolinite structure consists of alternating layers of silica tetrahedra and alumina octahedra. These twin layers are held together by hydrogen bonds.
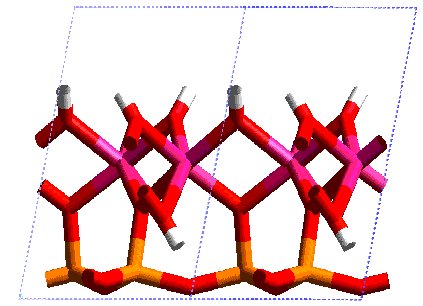
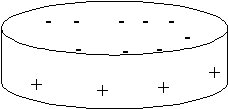
The particles are plate shaped and the surfaces possess charge. The basal surfaces are negatively charged due to isomorphous substitution of Si by Al and Mg for Al. At the edges the silica and alumina sheets are broken, exposing silanol and aluminol groups which become positively charged at low pH and negatively charged at high pH.
In order to keep the kaolinite particles dispersed during processing dispersants are often added. A typical dispersant is polyacrylic acid (PAA), an anionic polyelectrolyte. When PAA adsorbs on kaolinite the electrostatic repulsions between the particles increase and the dispersion becomes stable 1; which in turn leads to a lower relative viscosity. This project intends to use the technique of NMR to examine the relaxation rates of water at the kaolinite surface. When polymer adsorbs the solvent dynamics change, giving an insight into the adsorption process. Initially work will concentrate on the adsorption of PAA onto kaolinite as a function of pH and ionic strength.
1 The effect of polymer and surfactant adsorption on the colloidal stability and rheology of kaolin dispersions. Colloids and Surfaces A, 159, 199, 197-208.
This project is funded by the EPSRC and Imerys.