Colloid and Polymer interactions

Dong Qiu (previous postdoc in the group, left in 2006)
Colloidal dispersions are widely studied because of their theoretical and practical importance. They are used as model systems in simulating atoms or molecules' behavior, as building blocks for ordered structures and mostly as products in ordinary life for example ink, paint, cosmetics and so on. Recently I am interested in the areas listed below.
1. The effective pair potential between colloidal particles
Direct determination of this pair potential between two individual particles is difficult especially for those of 100 nm or less; therefore, more indirect methods are involved. Small-angle scattering techniques record the particle-particle correlation by a term of structure factor (Figure 1), which can be tracked back to the effective pair potentials.
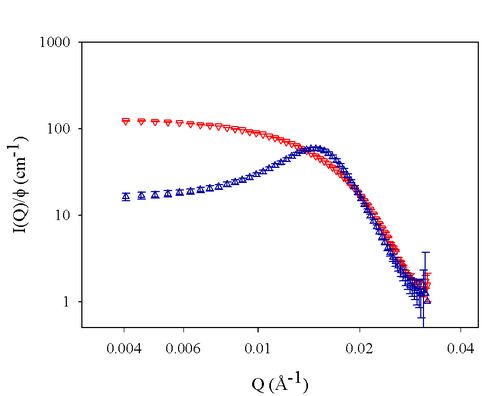
Figure 1. SANS spectra from ∇: Non-interaction system (dilute samples) Δ: interaction system (concentrated samples) Layer-contrast-matched: polymer layers are invisible.
2. Polymer stabilization of colloidal particles
Adsorbed polymers in good solvent can provide steric stabilizations. Such colloidal dispersions can stand for high ionic strength (Figure 2). The structure of the adsorbed polymer is closely related to the steric stability which can be studied by small-angle neutron scattering (SANS), PCS, NMR etc. The effect of ions on the polymer conformation at the surface is especially important as in practical most colloidal dispersions need to be used in an ionic environment. Our study aims to give instructions on how to manipulate the polymer adsorption and desorption as desired.

Figure 2. Klebosol 30R50 silica dispersions with different PEO concentrations in presence from left to right: 0, 0.35 mg.m-2, 0.71 mg.m-2 and 1.95 mg.m-2, 10 mM NaCl is present and the volume fraction of silica particles was 0.012.
3. The preparation of monodisperse polymeric latexes and their self-assembly
Through surfactant-free emulsion polymerization, monodisperse latex particles can be formed. They can be either stabilized by electrostatic repulsion or steric repulsion (for example by grafted PEO chains) or both. With “suitable” effective pair potentials, these particles tend to be self-assembled (Figure 3), which can be used as model systems to study the crystallization process.
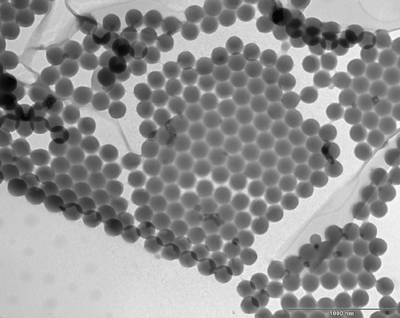
Figure 3. TEM image of grafted PEO-2000 stabilized polystyrene latex made by surfactant-free emulsion polymerisation.
The work has got financial support from Unilever and Eastman Kodak. ILL (Grenoble, France), ISIS (Didcot, U.K.), Juelich Research Centre (Juelich, Germany), SINQ (Switzerland) and NIST (Maryland, USA) are thanked for providing precious neutron beam times and support for access to these facilities.
Methods used: SANS, SAXS, Solvent Relaxation NMR, PCS, Rheology, TEM and SEM