Publications - 2004
Previous year | Overview | Next year
Impacting the Length of Wormlike Micelles Using Mixed Surfactant Systems
Vania Croce, Terence Cosgrove, and Cécile A. Dreiss
School of Chemistry, The University of Bristol, Cantock's Close, Bristol, BS8 1TS, United Kingdom
Geoff Maitland and Trevor Hughes
Schlumberger Cambridge Research, High Cross, Madingley Road, Cambridge CB3 0EL, U.K
Göran Karlsson
Department of Physical Chemistry, Uppsala University, Box 579, Uppsala S-75123, Sweden
Langmuir, 20(19), 7984 - 7990, 2004
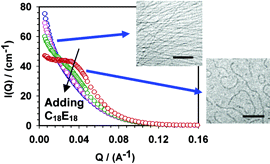
Abstract
The effect of adding an alcohol ethoxylate nonionic surfactant (C18E18) to aqueous solutions of a cationic surfactant, erucyl bis(hydroxyethyl) methylammonium chloride (EHAC, CH3(CH2)7(CH)2(CH2)12N+-(CH2CH2OH)2CH3Cl-), was studied using small-angle neutron scattering (SANS), steady-state rheology, and cryo-transmission electron microscopy (Cryo-TEM). This cationic surfactant has the ability to self-assemble into giant wormlike micelles in the presence of an electrolyte, such as KCl. In salt-free solutions, the mixture of the two surfactants gave rise to spherical micelles. The scattering curves obtained were fitted with a polydisperse core-shell model combined with a Hayter Penfold potential. The inner and outer radii were found to be dependent on the surfactant ratio. In the presence of KCl, mixed wormlike micelles were formed. However, further addition of C18E18 promoted the breaking of the micellar worms with the appearance of a structure peak in the scattering curves. In addition, it was found that the low shear viscosity is decreased upon addition of the alcohol ethoxylate nonionic surfactant. These findings are in good qualitative agreement with the Cryo-TEM images. The results show that the addition of the nonionic surfactant to the system is a method of controlling the worm length.
Dynamic Light Scattering Studies of Poly(ethylene oxide) Adsorbed on Laponite: Layer Conformation and Its Effect on Particle Stability
Andrew Nelson and Terence Cosgrove
School of Chemistry, The University of Bristol, Cantock's Close, Bristol BS8 1TS, United Kingdom
Langmuir (2004), 20(24), 10382-10388
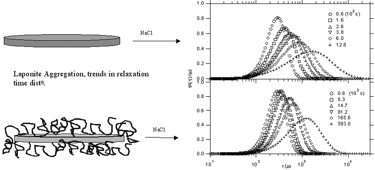
Abstract
Dynamic light scattering has been used to determine the hydrodynamic thickness of poly(ethylene oxide) (PEO) adsorbed on synthetic anisotropic clay particles (Laponite) as a function of molecular weight. The layer thicknesses, and their increase with molecular weight, indicate that the conformation of the adsorbed layer is very compact and is much smaller than those normally observed for polymer adsorption on flat interfaces. The aggregation kinetics of the polymer coated particles in 5 mM NaCl was analyzed in a quantitative manner, revealing that the potential barrier to aggregation is strongly enhanced when polymer is present.
Formation of a Supramolecular Gel between alpha-Cyclodextrin and Free and Adsorbed PEO on the Surface of Colloidal Silica: Effect of Temperature, Solvent, and Particle Size
CÚcile A. Dreiss, Terence Cosgrove, Francisco N. Newby
School of Chemistry, The University of Bristol, Cantock's Close, Bristol BS8 1TS, United Kingdom
Edvaldo Sabadini
Instituto de Quimica, Universidade Estadual de Campinas, Caixa Postal 6154, CEP 13084-862 Campinas, Brazil
Langmuir, 20(21), 9124 - 9129, 2004
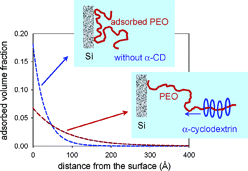
Abstract
Aqueous solutions of alpha-cyclodextrin (α-CD) complex spontaneously with poly(ethylene oxide) (PEO), forming a supramolecular structure known as pseudopolyrotaxane. We have studied the formation of the complex obtained from the threading of α-CD onto PEO, both free in solution and adsorbed on colloidal silica. The kinetics of the reaction were studied by gravimetric methods and determined as a function of temperature and solvent composition for the PEO free in solution. PEO was then adsorbed on the surface of colloidal silica particles, and the monomers were displaced by systematically varying the degree of complexation, the concentration of particles, and the molecular weight of the polymer. The effect of the size of the silica particles on the yield of the reaction was also studied. With the adsorbed PEO, the complexation was found to be partial and to take place from the tails of the polymer. The formation of a gel network containing silica at high degrees of complexation was observed. Small-angle X-ray and neutron scattering experiments were performed to study the configuration of the polymeric chains and confirmed the partial desorption of the polymer from the surface of the silica upon complexation.
Mixed Spherical and Wormlike Micelles: A Contrast-Matching Study by Small-Angle Neutron Scattering
Vania Croce, Terence Cosgrove, and CÚcile A. Dreiss
School of Chemistry, The University of Bristol, Cantock's Close, Bristol BS8 1TS, United Kingdom
Geoff Maitland and Trevor Hughes
Schlumberger Cambridge Research, High Cross, Madingley Rd, Cambridge CB3 0EL U.K.
Langmuir (2004), 20(23), 9978-9982
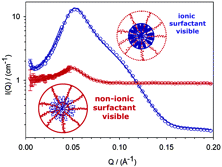
Abstract
Small-angle neutron scattering studies were used to investigate the effect of adding an alcohol ethoxylate nonionic surfactant (d-C12E20) to aqueous solutions of a cationic surfactant, erucyl bis(hydroxyethyl) methylammonium chloride (EHAC), with and without salt (KCl). The systematic use of contrast-matching, by alternately highlighting or hiding one of the surfactants, confirms that mixed micelles are formed. In salt-free solutions, mixed spherical micelles are formed and a core-shell model combined with a Hayter-Penfold potential was used to describe the data. The core radius is dominated by the EHAC tails and the outer radius determined by the ethoxylate headgroups of the nonionic surfactant. Addition of KCl promotes micellar growth; however, results of varying the solvent contrast revealed that when the nonionic surfactant is incorporated into the wormlike structure micellar breaking is promoted. Thus, mixed wormlike micelles with shorter contour lengths compared to the pure EHAC worms are formed.
PEO Penetration into Water-Plasticized Poly(vinylphenol) Thin Films
Chen Lu and Robert Pelton*
McMaster Centre for Pulp and Paper Research, Department of Chemical Engineering, McMaster University, Hamilton, ONT, Canada L8S 4L7
Robert Richardson
H. H. Wills Physics Laboratory, University of Bristol, Tyndall Avenue, Bristol, BS8 1TL U.K
Terence Cosgrove
School of Chemistry, The University of Bristol, Cantock's Close, Bristol BS8 1TS, United Kingdom
Kari Dalnoki-Veress
Department of Physics and Astronomy, McMaster University, Hamilton, ONT, Canada L8S 4L7
Macromolecules, 37(2), 494-500, 2004
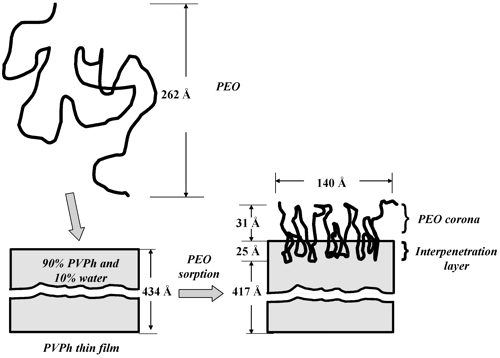
Abstract
Neutron reflectivity was used to characterize the adsorption of poly(ethylene oxide) (PEO), 150 kDa, on both polystyrene (PS) and poly(vinylphenol) (PVPh), thin films in water. The PVPh films had thicknesses of 434 and 438 Å and contained 9% v/v water in contrast to the 492 Å PS film which contained no water. PEO did not adsorb on the PS film, while the specific adsorption of PEO on the PVPh film was 1.2 mg/m2 at pH = 6.6. Reflectivity profiles from both deuterated and hydrogenated PEO in D2O provided a comprehensive picture of PEO on/in the PVPh film. The PEO distribution was modeled by two uniform layers. The external layer was a 30 Å thick corona of PEO (12.5%v/v) in water. Between the corona and bulk PVPh film was a 25 Å interpenetration layer consisting of 22% PEO, 76% PVPh, and 2% water. Since the interpenetration layer accounted for 45% of the bound PEO, it is proposed that about half of each adsorbed PEO molecule enters the PVPh film. Thus, the PEO interaction with a PVPh hydrogel can be considered as an example of a polymer/polymer complex formation leading to nanoscale PEO/PVPh blend coated with a water-swollen PEO corona.
A Small-Angle Neutron Scattering Study of Adsorbed Poly(ethylene oxide) on Laponite
Andrew Nelson* and Terence Cosgrove
School of Chemistry, The University of Bristol, Cantock's Close, Bristol BS8 1TS, United Kingdom
Langmuir, 20 (6), 2298-2304, 2004
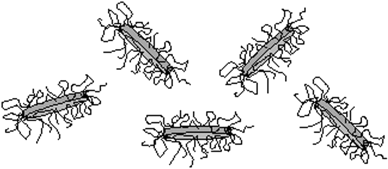
Abstract
The adsorption of poly(ethylene oxide) (PEO) on synthetic anisotropic clay particles (Laponite) has been investigated as a function of the molecular weight. Contrast variation small-angle neutron scattering (SANS) measurements were used to characterize the distribution and adsorbed amount of polymer on the particles. These experiments show not only that polymer is present on the face of the clay particle but that it also extends or "wraps" over the edges. The edge layer was thicker than the face layer for all the molecular weights studied. The polymer layers are unusually thin, with a thickness and adsorbed amount that show little variation with molecular weight.
Detailed Modeling of the Volume Fraction Profile of Adsorbed Polymer Layers Using Small-Angle Neutron Scattering
John C. Marshall, Terence Cosgrove, Timothy M. Obey, and CÚcile A. Dreiss
School of Chemistry, University of Bristol, Cantock's Close, Bristol BS8 1TS, U.K
Frans Leermakers
Laboratory for Physical and Colloid Chemistry, Agricultural University, Dreyenplein 6, 6703 H B Wageningen, The Netherlands
Langmuir, 20 (11), 4480-4488, 2004
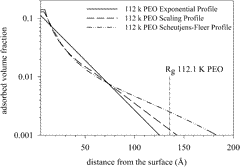
Abstract
A detailed analysis of on and off-contrast small-angle neutron scattering from poly(ethylene oxide)s adsorbed on polystyrene latex is presented. The results have been fitted to an exponential decay, self-consistent mean-field (SCF) and scaling models of the volume fraction profile. As the chain length increases a clear self-similar layer emerges in both the SCF and scaling profiles. The RMS thickness of the adsorbed layer in the plateau of the adsorption isotherm varies as M0.4 for both the SCF and scaling profiles but the exponential one however gives a much lower exponent consistent with the neglect of the tail or distal region. The new constrained version of the Scheutjens-Fleer model was able to predict the structure of the layer and hence the scattering with great accuracy.
Rheology, cryogenic transmission electron microscopy, and small-angle neutron scattering of highly viscoelastic wormlike micellar solutions. [Erratum to document cited in CA139:312831]
Vania Croce, Terence Cosgrove, Geoff Maitland, Trevor Hughes and Göran Karlsson
School of Chemistry, University of Bristol, Bristol, UK
Langmuir (2004), 20(1), 277
Abstract
In the title, "cryogenic transmission electron spectroscopy" should read "cryogenic transmission electron microscopy".
Previous year | Overview | Next year