Studies of Inclusion Complexes of Polymer with Cyclodextrins

Wirach Taweepreda (previous student, left the group in 2005)
Current Contact address:
Hat-Yai, Songkla 90112, THAILAND
Tel.: +66-74-288361
E-mail: twirach@yahoo.com
Polymer Science Program
Faculty of Science
Prince of Songkla University
Introduction
Cyclodextrins (CDs) and their chemically modified derivatives have been the subject of numerous investigations. We making the complexes of polymers with cyclodextrins that can imporve their performance, and consequently, the physicochemical properties have been changed. For example in principle it is possible to make water insoluble polymers water soluble and those that are surface active, inactive etc. The formation of inclusion complexes were investigated and presented the 2 types of threading model: hydrophilic and hydrophobic polymers. The compounds were isolated and characterised by using Solid-State CP/MS 13C-NMR, Powder X-ray Diffraction Pattern, EM, AFM and SANS.
This project is sponsored by Thai Government.
Research
Cyclodextrins (CDs) (figure 1) and their inclusion complexes (ICs) (figure 2) with polymer have been the subject of numerous investigations. The inclusion complexation of CDs with a polymeric material is partly driven by intermolecular hydrogen bonding between neighbouring CDs. The important factor in the ICs formation is the size matching between the cross-sectional area of polymeric guest and the internal CD's cavity. Inclusion complexes of polymers with normal CDs often reduce the solubility of polymer leading to phase separation. Hydroxypropyl substituted CDs (HP-CDs) however will form a polypseudorotaxane but with a higher solubility. HP-CDs spontaneously form a polypseudorotaxane with block copolymers and their micelles. In these experiments, the inclusion complexes of linear (PEO and PDMS) or non-linear polymers (PDMS-b-PEO, PEO-PPO-PEO, PPO-PEO-PPO, and star-PEO) with normal or hydroxypropyl derivative CDs were prepared and investigated by using nephelometer, visual observation with a digital web cam, high resolution and pulse field gradient 1H-NMR spectroscopy, solid-state CP/MAS 13C-NMR spectroscopy, powder X-ray diffraction, scanning electron microscopy (SEM), atomic force microscopy (AFM), and small-angle neutron scattering (SANS). The results suggested that the polymers are size selective, as α-CD or HP-α-CD can complex with only the PEO, HP-β-CD with PPO whereas the γ-CD or HP-γ-CD form can complex with 2 PEO chains, PPO and PDMS.
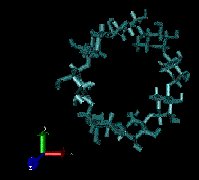
Figure 1: Molecular dynamics of γ-CD in water box (simulated using CHARMM).
Press on image to see animation (file size: 1.7 MB).
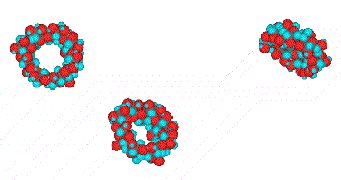
Figure 2: Animation of a pseudopolyrotaxane formation between PDMS with γ-CD.
Press on image to see animation (file size: 0.7 MB).
Publications
Sabadini, E.; Cosgrove, T.; Taweepreda, W.;
Complexation between alpha-Cyclodextrin and Poly(ethylene oxide) Physically Adsorbed on the Surface of Colloidal Silica
Langmuir (Article); 2003 19 (11); 4812-4816
Links
Conversion of UNITS
- http://www.sciencemadesimple.com/conversions.html
- http://students.washington.edu/kyle/temp.html
- http://www.chemie.fu-berlin.de/chemistry/general/units_en.html
- http://www.onlineconversion.com/
Material Safety Data Sheets
Techniques - NMR Techniques
- http://www.cis.rit.edu/htbooks/nmr/
- http://drx.ch.huji.ac.il/nmr/techniques/1d/multi.html
- http://www.spectroscopynow.com
- http://www.chem.unl.edu/nmrweb.html
- http://www.york.ac.uk/depts/chem/services/nmr/
- http://www.ncl.ac.uk/garnet/nmr/
- http://www.biochem.ucl.ac.uk/bsm/nmr/
Techniques - SANS Techniques