CAUSES
There is an annual variation in the concentration of ozone in the stratosphere, illustrated by the formation of the Antarctic ozone hole.
Dynamics
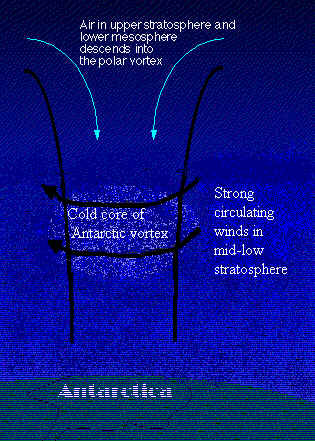
During the winter the Antarctic stratosphere is in polar night for three months of the year, causing the air to cool and descend. A vortex forms with a core of very cold air; the temperature can fall to below 190K. This low temperature allows the formation of polar stratospheric clouds, PSCs.

Air from above the vortex (altitude, 40km), containing HCl and ClONO2, is drawn down through the cold core, ending at approximately 20km above the Earth's surface.
Chemistry
Usually most of the chlorine in the stratosphere is bound up in the reservoir compounds (HCl and ClONO2) as a result of the termination reactions.
Cl + CH4 ® HCl + CH3
ClO + NO2 ® ClONO2
During the polar night, these chlorine reservoir compounds are converted to active forms by heterogenous processes which occur on PSC surfaces.
ClONO2 + HCl ® Cl2 + HNO3
Cl2 is released, whereas HNO3 remains in the ice particles that make up the PSCs. When the Sun returns in the spring, this Cl2 is readily photolysed and Cl is released.
Cl2 + hu ® Cl + Cl
The normal catalytic cycle
Cl + O3 ® ClO + O2
ClO + O ® Cl +O2
does not occur at this lower altitude (~20km), as the concentration of oxygen atoms is too low. Instead, there is dimer formation:
Cl + O3 ® ClO + O2
ClO + ClO + M ® Cl2O2 + M
Cl2O2 + hu ® Cl + Cl + O2
This is a catalytic cycle for which the normal termination reactions are not efficient.
Cl + CH4 ® HCl + CH3 This requires high temperatures which are not present.
ClO + NO2 ® ClONO2 There is very little NO2 as it is in the form HNO3 and is incorporated into the PSCs.
Huge ozone losses take place until the vortex warms up and breaks down, thus allowing NOx to mix in from outside.
It has been found that there has been a decline in springtime ozone concentrations since the late 1970s. This is partly due to the release of CFCs into the atmosphere.
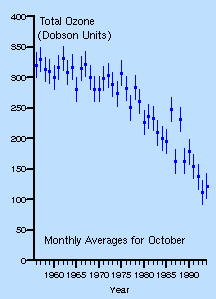 Ozone
Depletion (image taken from
http://www.atm.ch.cam.ac.uk/tour/part1.html)
Ozone
Depletion (image taken from
http://www.atm.ch.cam.ac.uk/tour/part1.html)
A similar scenario is seen in the northern hemisphere. A significant difference is the size of the ozone hole which is smaller due to the higher temperature. This leads to less PSC formation, hence lower levels of active Cl in the atmosphere.