 Batteries
Batteries
Imagine life today
without the battery, no cars, no mobile phones, no torches, no walkmans, in
fact no portable electrical appliances at all!!!!!!!!!!!!!!!! In fact batteries
have been produced commercially for over a century and there is a wide range of
power and size, from 0.1Wh miniature cells to 10MWh load levelling batteries.
The first battery was made by Volta in 1800.

A battery is a
cell, which converts chemical energy into electricity. The electron transfer
that occurs in a redox reaction is transferred into an electric current.
A common battery is the Alkaline battery i.e. Duracell battery. This battery consists of a Zinc electrode and a Manganese oxide electrode and an alkaline electrolyte.
The redox reaction that the battery relies on is the oxidation of the Zinc and the reduction of the manganese:
Zn ® Zn2+ + 2e-
MnO2 + 2H2O + 2e- ® 2MnOOH +2OH-
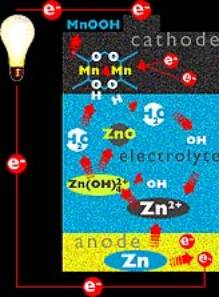
The alkaline cell was first developed during the 1950’s but is closely based on the Leclanche cell that was developed during 1870-1890. The major difference between the alkaline and Leclanche cell is that the alkaline cell has an improved electrolyte, aqueous KOH.
The Leclanche cell had the over discharge of:
Zn + 2MnO2 + 2H2O + ZnCl2 ® 2MnOOH + 2ZnOHCl
There are 2 main
types of battery
- Primary cell: use once and then discard.
- Leclanche cells
- Alkaline cells
- Lithium batteries
- Secondary cell: rechargeable as redox reaction is reversible.
- Pb/Acid
- NiCd
- Lithium ion
A common use of secondary cells is the car battery, which is used for starting the internal combustion engine. The car battery is rechargeable and has lead and lead oxide electrodes and a strong acid electrolyte.