If you peel or chop an onion, often your eyes begin to water. What causes this? It seems obvious - something in the onion causes it. But an uncut, unpeeled onion is not lachrymatory (meaning a substance which causes crying) and has almost no odour. So the substance that causes crying must be produced only upon peeling or chopping. Similiarly in garlic - the distinctive smell of garlic only occurs when the garlic is chopped, peeled or crushed. There is some biochemical mechanism that produces these substances only after peeling or chopping.
Theodor Wertheim, a German chemist was the first to carry out a chemical study of garlic. In 1844 he extracted 'garlic oil' from garlic by steam distillation. He commented on the sulfurous nature of this oil, and named the hydrocarbon group present 'allyl'. This name is still in use today, it describes the group CH2=CH-CH2-.
Semmler in 1892 identified a component of the oil as diallyl disulfide:
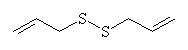
He also extracted onion oil, and identified it as containing, among other things, propanal:
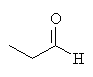
Neither of these chemicals cause the effects described above. What's going on? The substance responsible for the properties must be sufficiently unstable to decay to the above products when subjected to the harsh conditions of steam distillation. Cavallito and others working in the US in 1944 extracted compounds from garlic using gentler conditions than steam distillation. He found that certian compounds predominated under certain conditions:
| method of extraction from garlic | compound extracted (click for 3-D!) |
|---|---|
| steam | 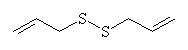 diallyl disulfide diallyl disulfide
|
| ethanol and water, room temp | 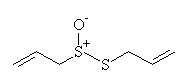 allicin allicin
|
| cold ethanol | 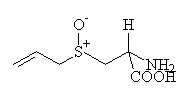 alliin alliin
|
The substance responsible for the smell of garlic is allicin, and it is formed from alliin when the garlic is cut or crushed. Alliin is an amino acid based on cysteine, and it has 4 stereoisomers (exciting chemical fact! for those who are interested.) Only one isomer is present in garlic.
Onions were investigated in a similiar way by the Finnish Nobel laureate Artturi Virtanen. He applied similiar extraction methods to those used by Cavallito to onions and found the following:
| method of extraction from onion | compound extracted (click for 3-D!) |
|---|---|
| steam |  propanal propanal
|
| freon and water, cold | 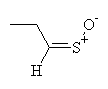 syn- lachrymatory factor syn- lachrymatory factor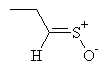 anti- lachrymatory factor anti- lachrymatory factor
|
| cold ethanol | 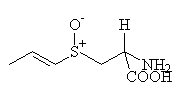 lachrymatory precursor lachrymatory precursor
|
The lachrymatory factor is the compound that causes crying when onions are peeled. It has two forms, termed syn and anti (these names correspond to the different arrangements of the bonds in space). Notice that the lachrymatory precursor differs from alliin only in the position of the carbon-carbon double bond.
Further work has lead to the discovery of the reactions that occur in cut garlic and onions, and the next page will explain....
Click here to continue..| introduction | alliums: their curious properties | a little bit of chemistry |
| Medicinal properties and a bit more chemistry | references and further information | links |