Auroral Colour Composition is the Atmosphere's Fingerprint
Auroral colour is composed of lines and bands in the visible, ultra-violet and infra-red areas of the spectrum. Light is emitted from the gases in the atmosphere which, at typical auroral altitudes, are atoms and molecules of nitrogen and oxygen. The lines in the spectrum come from atoms, whereas molecules cause the bands.

The reason that gases emit light can be
explained in terms of their atomic structure.
An atom consists of a positive nucleus with one or more electrons circulating it in
fixed orbits. When auroral particles-free electrons and protons-enter the
atmosphere, the collide with the gas particles in the air and are slowed
down. The northern lights are a direct result of these collisions.
In physical terms what happens is that the auroral particles collide with the
atoms and molecules in the atmosphere, and the electrons that move round the
atoms' including their nuclei have their orbits disturbed. This may result
in one of two things:
-the electron may fly away from its nucleus at great speed
leaving the
atom or molecule electrically charged (ionized).
-the electron may begin to move around the nucleus in a new
orbit.
The electron is said to have "jumped" from one orbit to another and
the entire particle has received an excess of energy; it has become excited.
An excited particle is unstable. In a millionth of a second it will normally release its excess energy. One of the ways that this can happen is that the electron falls back to its original orbit-its ground state-while its excess energy is given off in the form of a little flash of light (a photon). When there is a vast number of these small flashes, as during the northern lights' display, the cumulative effect of them creates what is perceived as a luminous formation in the sky.
The wavelengths of the various lines and bands in the light emitted from the northern lights can be compared with the known spectra of different atmospheric gases. In this way, the gases giving rise to the light can be identified. The colour of each proton is dictated by the gas particle that it comes from and the state that the particle was in. An atom, molecule or ion that has been excited to a certain level, emits photons of a definite wavelength or colour.
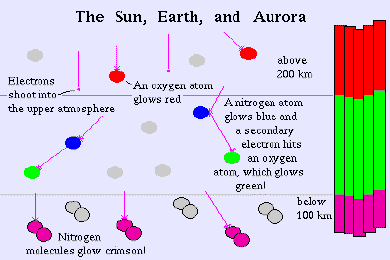
High-altitude oxygen, about 200 miles up, is the source of the rare, all-red auroras. Oxygen at lower altitudes, about 60 miles up, produces a brilliant yellow-green, the brightest and most common auroral colour. Ionized nitrogen molecules produce blue light; neutral nitrogen glows red. The nitrogen's create the purplish-red lower borders and ripple edges of the aurora.
The colour composition of the northern lights therefore provides information about the ingredients of the gas it emanates from, in other words the earths atmosphere. Thus it is possible to call the northern lights the "atmosphere's fingerprint".
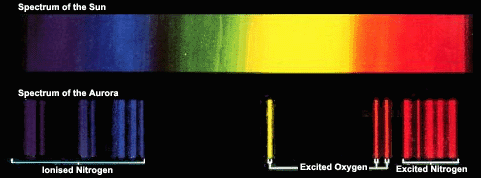
The strength of the various lines or bands and how they appear in relation to one another can reveal information about the amount of the different gas particles present in the atmosphere. If there are only a few particles of one type of gas, there should be a weaker light from it, if there are many particles of another, the light emanating from it should be strong.