
Lavoisier started his research on the products of Calcination
with the help of a three figure balance. He was the first to recognise the
importance of precision and thanks to his thorough work, he was able to make
revolutionary discoveries in the world of science.
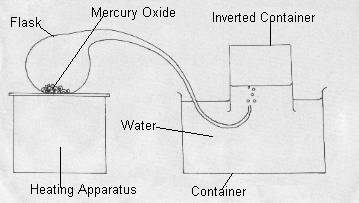
Lavoisier started by using mercury. When heated, mercury formed mercury oxide, in the form of small red balls. Lavoisier was interested in the composition of this red solid. Therefore he tried to reduce the mercury oxide: Lavoisier placed an ounce of mercury oxide and 48 grains of coal in a round-bottomed flask with the neck of the flask extending into a tube. This mixture was heated. The end of the tube from the flask
was placed into a bowl of water with the opening of the tube directly underneath a semi-submerged bell jar. As gas is produced in the flask, the gas travels through the tube and into the bell jar, thus displacing the water in the bell jar. The water level at the start and end of the experiment was measured.
According to his scientific observations, a colourless gas was released. The most important property of this gas was that a candle extinguished in its presence almost instantaneously. Also, it reacted with Lime Water to form a cloudy solution. These properties were characteristic of 'fixed air'- a gas which we now call Carbon Dioxide.
Lavoisier decide to repeat the same experiment but this time, with no
Coal. It took him a several hours before the mercury oxide had been
completely reduced (shown below). However, this time the gas released did not react with
Lime Water and the gas could be used for calcination. Furthermore, candles
burnt with a very intense flame-brighter than in normal air. He initially
named this gas 'respirable air' and then later on 'oxygen'.
Given the fact that in the first experiment the coal had completely disappeared and that fixed air had been produced, whereas in the second experiment only respirable air was produced, Lavoisier concluded that fixed air was composed of respirable air and coal.
However, Lavoisier needed more experimental data to explain the release of respirable air in the second experiment.
For his other experiments on calcination, Lavoisier chose phosphorus as the element burns readily and produces phosphoric acid.
Having placed 1/2 g of phosphorus in an open flask, he weighed the flask and phosphorus together and then placed the flask under a bell jar, partially submerged in a bowl of mercury.
After having lit the phosphorus, Lavoisier noted that the mass of the flask had increased by 0.3g whereas the volume of air in the bell jar had increased by 0.3L, leaving the bell jar with a higher percentage of fixed air than normal air. Therefore it follows that the air in the bell jar was fixed by the phosphorus and that this fixation explains the increase in mass of the flask and its content.
Therefore a certain percentage of the air can be fixed. This particular type of air, respirable air, was released by the mercury oxide in the second experiment. Lavoisier concluded from this that Calcination consisted of the fixation of air as opposed to the release of Phlogiston. It was by questioning the existence of Phlogiston that Lavoisier was able to prove Stahl's theory wrong. He also showed that the air in the atmosphere consisted if at least 2 gases: fixed air and oxygen.