Lavoisier had now established the fact that oxygen was a gas present in normal air and that it was responsible for the effects of combustion on metals, sulphur, phosphorus and many other substances.
Lavoisier continued his research on combustion. He placed oxygen and hydrogen in a glass balloon and lit the mixture. Lavoisier observed an explosion and the release of a colourless gas which rapidly condensed against the sides of the container, producing a colourless liquid.
The liquid produced was water. Lavoisier concluded that the combustion of hydrogen produced water but he wanted more results to support his new discovery. For this, he had to come up with a new experiment.
The new experiment involved passing vapour over incandescent iron filings. His other experiments showed that mercury oxide could decompose on heating and he hoped that heat would have a similar effect on water. The experiment worked and the two gases were collected over mercury: hydrogen and oxygen.
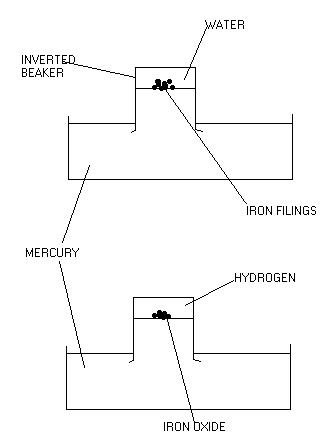
However, Lavoisier wanted to establish the exact quantity of oxygen and hydrogen required to produce water. Lavoisier chose to use iron again : he filled a bowl and a small beaker with mercury, then placed a small quantity of water and iron filings into the small beaker (Figure 2). The beaker was inverted and placed into the bowl. The iron filings floated on the surface of the mercury as the iron is less dense then the mercury.
After a few days, Lavoisier found that the iron had reacted with the water, producing a reddish powder (iron oxide) and a small quantity of hydrogen. Lavoisier noted the volume of hydrogen gas produced.
Fe + H2O ----------------> FeO + H2
Having noted the volume of gas produced in the previous experiment with the decomposition of water vapour, Lavoisier concluded that water was composed of two-thirds hydrogen and one third oxygen.

Figure 2
He concluded this by examining the results of his experiments: He used the same volume of water in the two experiments with water and found that 'x' litres of gas (a mixture of oxygen and hydrogen) were released on decomposing water vapour over hot iron. He also found that only 2/3 x litres of hydrogen were released when reacting iron with water. Lavoisier had discovered in a previous experiment that water was composed of hydrogen and water, it therefore follows that 1/3 x litres of oxygen must have been released and reacted with the iron. Thus Lavoisier discovered that water consisted of one part oxygen and two parts hydrogen.
The discovery of the decomposition of water contradicted once again 'Aristotle's Four Elements' and proved that water was composed of two elements called oxygen and hydrogen.