nuclear
fission ![]()
In heavy, large nuclei - such as uranium - there is a balance between the attractive force of electrons to nuclei and the repulsive force of the electrons. The increased repulsion, compared to smaller elements with fewer electrons, causes a reduction in the binding energy. This indicates it is now favorable for a heavy nucleus to split into two fragments, or transmute, forming two lighter nuclei.
238U ----> 119Pd + 119 Pd is an example of symmetric fission, however this is quite unusual. More common is decay by alpha emission, this usually releases large amounts of energy and forms unstable products. These fragments then undergo a series of radioactive decays involving the ejection of neutrons, beta rays and gamma rays.
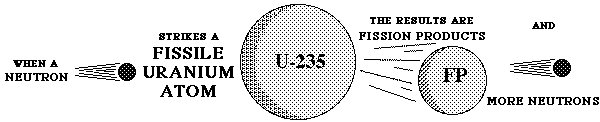
If uranium is exposed to a stream of neutrons a chain reaction may occur;
neutron + 238U ----> 145Ba + 94Kr. This can release a further two or three neutrons which may all cause further nuclear fission. Conditions for this type of chain reaction are very favorable with 235U.