TNT
Structure

Properties
Appearance Pale yellow solid
Molecular weight 227.1
Melting temperature 80.8° C, low, good for casting
Thermal ignition temp 300° C
Stability Chemically and thermally stable
Solubility Almost insoluble in water, sparingly soluble in organic liquids
Sensitivity Low sensitivity to impact and friction
History
1863 TNT first prepared by a German named Wilbrand.
1870 The 2,4,5 isomer was discovered and a study of the more commonly known 2,4,6-trinitrotoluene was carried out.
1891 Manufacture of TNT started in Germany
1902 TNT replaced picric acid and soon became the standard explosive for both sides during the Great War.
During the development of TNT, explosive mixtures, which included ammonium nitrate and/or aluminium, were also created to meet demands for more powerful and increased amounts of explosives.
Manufacture
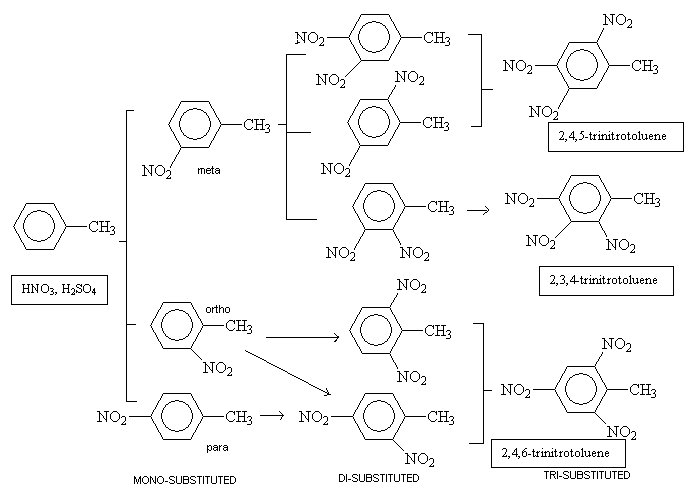
Trinitrotoluene is prepared from toluene, which is readily available from coal tar (a faction of oil, which can be separated by fractional distillation). Toluene is mixed with nitric and sulphuric acids so that electrophilic substitution of NO2 groups, commonly known as nitration, can occur. The mono-substituted product is formed first and then with increased reaction temperatures and acid concentrations the di-substituted and then the tri-substituted products are formed. The last step is often carried out with free SO3 in the mixture too. The progression below shows the steps and resulting isomers involved.
In industry, this reaction is carried out as a continuous process where reagents are added, products removed, but the reaction never stops. In order to end up with pure 2,4,6-trinitrotoluene, a pH9 4% sodium sulfite solution is used which attacks the unsymmetrical isomers of TNT resulting from the meta compound in the first substitution. These by-products can then be taken out from the mixture using an alkaline solution. If TNT was not pure and contained a mixture of isomers, it would be more sensitive and have a lower melting temperature.
Decomposition Products
If TNT is able to undergo full oxidation once it has been detonated, the products formed will be carbon dioxide, water and nitrogen gas. However, the oxygen content of the molecule is very low compared to the about of oxygen needed to form all of these products so extra oxygen is needed, i.e. combustion needs to occur. If this is not possible, as it would be in a confined space, the following reaction can occur.
The unoxidised carbon formed causes the sooty blasts that can often be seen after a bomb containing TNT has exploded. It is only after an explosion, when the reaction products come in contact with oxygen that full combustion can occur. This gives rise to heat released by explosion, and then extra heat released through combustion afterwards.