Cyanine
The search
for a suitable dye with the right thermal characteristics and chemical stability
lead first to polymethine (or cyanine) dyes:
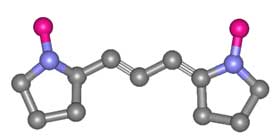
The basic polymethine chain is nothing more than a conjugated carbon chain,
but the dyes usually have a head group on either end of the chain. This head
group is typically heterocyclic, containing a sulphur or nitrogen atom.
These have varying absorption wavlengths from the UV to the nIR (near Infra Red). The wavelength of this transition increases by around 100nm for every extra vinyl unit in the chain. A polycyclic head group after the heterocycle can also affect the wavelength. The napthalene derivative B has a 300nm longer transition than th type A for the same length vinyl chain
| Type A head group (Chime Model) | Type B head group (Chime Model) |
These dyes often become unstable as the chain length increases sufficiently for the requisite 820nm nIR transition, so long term data storage using simple cyanines is difficult. Metal stabilisation of ionic cyanines has been employed to resolve this limitation and has to some extent been successful.