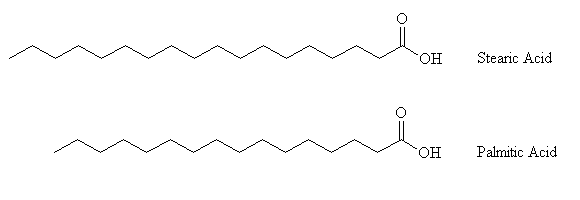
Candlesticks have been found dating from 3000 BC from Egypt and Crete. Candles are believed to be one of the first inventions of the ancient world. The candles used by the Romans were made from beeswax. Tallow candles were introduced during the twelfth century. Tallow is a hard fatty substance, which is usually obtained by rendering the suet of sheep or cattle. In 1825 M. E. Chevreul and J. L. Gay-Lussac obtained a patent for candles made of stearine, a mixture of stearic acid (melting point 69 ºC) and palmitic acid (melting point 61ºC). Modern candles are made from a mixture of hard paraffin, derived from petroleum, (melting point 51-55 ºC) and stearine. Stearine is added to a candle to increase the melting point and to prolong the burning time.

Candlewicks are made of plaited cotton yarn impregnated with boric acid, ammonium chloride and phosphates, these components reduce the smoke output and increase the burn time of the wick.
When lit, heat from the flame liquefies the wax near the base of the wick. The liquid flows upward by capillary action, and is then vaporized by the heat. The flame is the combustion of the wax vapour.
 In the region surrounding the burning wick the flame forms a dark cone
topped by a luminous yellow region. At the bottom of the flame there is
a light blue reaction zone. The temperature in the dark cone near the wick
is fairly low, 600 ºC, and rises to about 1,200 ºC in the centre
of the yellow region. The hottest part of the flame lies just off centre
on the edge of the yellow portion, and has a temperature of 1,400 ºC.
In the region surrounding the burning wick the flame forms a dark cone
topped by a luminous yellow region. At the bottom of the flame there is
a light blue reaction zone. The temperature in the dark cone near the wick
is fairly low, 600 ºC, and rises to about 1,200 ºC in the centre
of the yellow region. The hottest part of the flame lies just off centre
on the edge of the yellow portion, and has a temperature of 1,400 ºC.
The long chain hydrocarbon molecules are decomposed near the burning
wick in a stepwise manner, forming CH2 or C2H4
radicals. The light blue portion at the bottom of a flame is a reaction
zone. In this region C2 and CH are produced in excited states
and it is their respective band emissions that account for the blue colour.
The reaction zone extends to the yellow zone of the flame. It is here that radicals from the decomposed hydrocarbons react with oxygen from the air to form CO2 and water in a mechanism that is still not well understood. The unreacted fuel and the oxygen environment do not come into physical contact as a layer of combustion products separates them.
The luminous yellow part of the flame accounts for most of the light that is produced by the candle. This region is known as the carbon zone and consists of hot, carbon rich soot particles formed at the tip of the dark region from decomposed hydrocarbons. The soot particles cluster together to form chain aggregates and are heated to incandescence by thermal transfer from the reaction zone. Though the full visible spectrum is emitted, emissions from the yellow region are the most intense. However only 0.4% of the candle's energy is emitted in the visible region, most of the energy is emitted as photons from the infrared region, this accounts for the candles warmth.