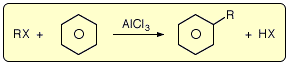
There are many different electrophilic substitution reactions associated with benzene and benzene derivatives. However, very few of them involve the formation of a carbon-carbon bond, one of the primary challenges in organic chemistry. In principle, reactions could be carried out with benzene in the presence of a sufficiently electrophilic carbon-based electrophile. There are two such transformations, known as the Friedel-Crafts reactions. The secret behind the success of both processes is the use of a Lewis acid, usually aluminium chloride. In the presence of this reagent, haloalkanes attack benzene to form alkylbenzenes.

Born:- March 12, 1832, Strasbourg,
Fr.
Died:- April 20, 1899, Montauban
Professor Charles Friedel was a French organic chemist and mineralogist who
discovered in 1877 the chemical process known as the Friedel-Crafts
reaction.
In 1854 Friedel entered C.A. Wurtz's laboratory and in 1856 was
appointed conservator of the mineralogical collections at the Superior National
School of Mines. In 1871 he began to lecture at the École Normale and in 1876
became professor of mineralogy at the Sorbonne, but on the death of Wurtz in
1884 he exchanged that position for the chair of organic chemistry.
He collaborated in efforts to form diamonds artificially,
studied the pyroelectric properties of crystals, determined crystallographic
constants, and did research on ketone and aldehyde compounds. Friedel was
the chief founder of Revue Générale de Chimie in 1899.
Click to enlarge
Professor James Mason Crafts was an American organic chemist who studied and worked at the Massachusetts Institute of Technology, Cambridge, Massachusetts USA. As Charles Friedel's younger and less experienced colleague, he assisted in the development of the reaction to which they gave their names.
Click to enlarge
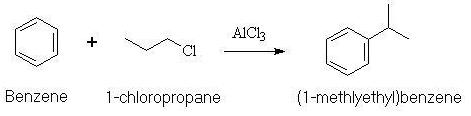
In 1877, Friedel and Crafts discovered that a haloalkane reacts with benzene in the presence of an aluminium halide. The resulting products are the alkylbenzene and hydrogen halide. This reaction, which can be carried out in the presence of other Lewis Acid catalysts, is called the Friedel-Crafts alkylation of benzene.
The reactivity of the haloalkane increases with the polarity of the C-X bond (Cl in the case above) in the order RI<RBr<RCl<RF. The aluminium chloride above could be any aluminium halide or a number of other compounds. These are all Lewis Acid catalysts.
With primary halides, the reaction begins with coordination of the Lewis acid to the halogen of the haloalkane. This coordination places a partial positive charge on the halogen-bearing carbon, rendering it more electrophilic. Attack on the benzene ring is followed by proton loss giving the observed product.
With secondary and tertiary halides, free carbocations are usually formed as intermediates.
Intramolecular alkylations can be used to fuse a new ring onto the benzene nucleus.
Friedel-Crafts alkylations can be carried out with any starting material, such as an alcohol or alkene, that acts as a precursor to a carbocation.
In summary, the Friedel-Crafts alkylation produces carbocations capable of electrophilic aromatic substitution by formation of aryl-carbon bonds. Haloalkanes, alkenes, and alcohols can be used to achieve aromatic alkylation in the presence of a Lewis acid.
Limitations of Friedel-Crafts Alkylation
The above reaction is accompanied by two important side reactions, one is polyalkylation (more than one alkyl group attaches itself to the ring); the other is carbocation rearrangement (The intermediate carbocation rearranges and so a different product is formed). This leads to a decrease in useful yield and a mixture of products which are difficult to seperate.
Because of these limitations, Friedel-Crafts alkylations are used rarely in synthetic chemistry. However a second reaction, again developed by Friedel and Crafts, hepled to fix this problem. It involves an electrophilic carbon species that does not rearrange and, moreover, one that deactivates the ring to prevent further substitution(polyalkylation).
This reaction proceeds through the intermediacy of acylium cations. These ions readily attack benzene to form ketones.
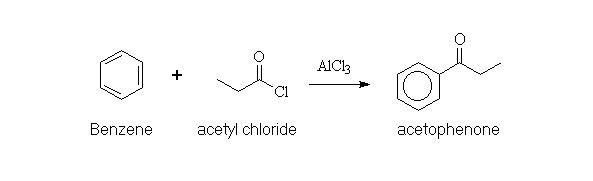
Benzene reacts with alkanoyl (acyl) halides in the presence of an aluminium halide to give 1-phenylketones (phenyl ketones). An example is the preparation of 1-phenylethanone (acetophenone) from benzene and acetyl chloride, by using aluminium chloride as the Lewis acid.
Alkanoyl (acyl) chlorides are reactive derivatives of carboxylic acids. They react with Lewis acids to produce acylium cations. The acylium ion is sufficiently electrophilic to attack benzene by the usual aromatic substitution mechanism. Because this ion (now a substituent of the benzene ring) is electron withdrawing, it deactivates the ring and protects it from further substitution. The effect is accentuated by the formation of a strong complex between the aluminium chloride catalyst and the carbonyl function of the product ketone.
This complexation removes the aluminium chloride from the reaction mixture. Aqueous work-up is necessary to liberate the ketone from it's aluminium chloride complex.
In summary, problems encountered with Friedel-Crafts alkylation are avoided in Friedel-Crafts alkanoylation, in which an alkanoyl halide or carboxylic acid is the reaction partner, in the presence of a Lewis acid. Intermediate acylium cations undergo electrophilic aromatic substitution to obtain the resultant aromatic ketones.
Summary
A diagram summarizing both reactions:-
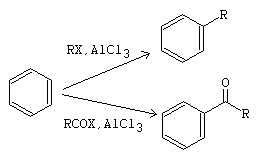
The top reaction is alkylation, the bottom reaction is alkanoylation.
Aluminum chloride has a central aluminum atom which has only 6 electrons; like boron in the acidic BF3, it is very electron-deficient. It is one of the stronger and more common Lewis acids used in chemistry. In the presence of alkyl halides (tertiary best) or acyl halides, it can remove the halide to generate a carbocation and AlCl4-. The carbocation will react with whatever is available, like an aromatic ring. With carbons that are less able to support a positive charge, the aluminum chloride just loosens the C-Cl bond enough to make the carbon positive enough to react with the aromatic ring (and to rearrange as if it were a carbocation). Aluminum chloride is a powerful dehydrating agent as well, although not very selective; partially hydrated (to reduce its acidity) it works very well as commercial dehydrating agent - the antiperspirant aluminum chlorhydrate.
FeCl3
Ferric chloride is the catalyst of choice for halogenating benzene rings. The reaction proceeds like that of alkyl halides with aluminum chloride, namely polarization to remove a halide to form FeCl4-, and thus generating Cl+. An easy way to do this reaction is just to add powdered iron to the reaction and an excess of halogen; the halogen oxidizes the Fe to FeX3 (where X could be other halogens as well) which then catalyzes the reaction.
Click on these to go to the links (some of which I have used to write this web site without permission):-
Reactions of organic compounds from Towson University
French page about Charles Friedel
The Bristol University homepage
Other information used on this page comes from:-
Organic Chemistry:- Vollhardt and Schore