SYNTHESIS:(1)
The high strain that the cubane framework is under has already been highlighted. The researchers had to very cautiously attach a nitro group to each of the corners of the cube in order to make the desired product. The insertion of the first four nitro groups could be done by manipulating functional groups:
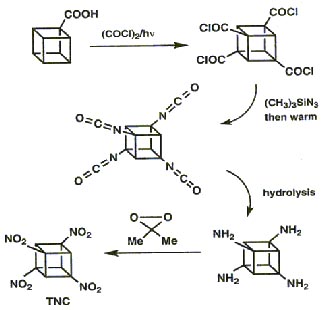
The key intermediate, cubane-1,3,5,7- tetracarboxylic acid (TNC), was obtained by clever application of the Brown-Kharasch photochlorocarbonylation to cubane mono-acid.
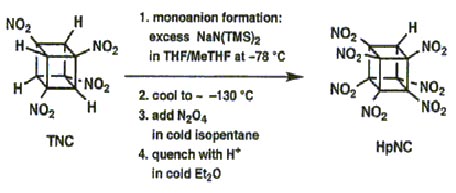
The addition of four further nitro groups proved far more difficult and new methodologies had to be developed, specifically the process of interfacial nitration. This method was used successfully to convert the sodium salt of TNC to pentanitrocubane (PNC) and then hexanitrocubane (HNC), both are stable materials.
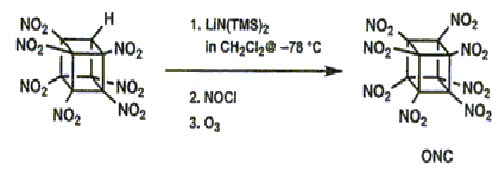
Interfacial nitration, however, proved deficient for further nitration of HNC and again new experimental methodology had to be developed for its successful conversion to heptanitrocubane (HpNC):
Addition of excess NOCl to a solution of the lithium salt of HpNC in dichloromethane at -78°C gave the long-sought ONC: