Honey is a saturated or super-saturated solution of sugars, the majority, approximately 84%, being a mixture of fructose and glucose. The super-saturated nature of the solution means that the water content is very low, usually only 15-21% by weight. Honey also contains a small amount of numerous other types of sugars as well as acids, proteins and minerals. Sugars are also called sweet carbohydrates and as such are composed of carbon (carbo-), hydrogen and oxygen (-hydrate). Carbohydrates are described by the number sub-units they contain. Glucose and fructose are monosaccharides, or simple sugars:
The difference between the two structures is determined by the position of the carbonyl functionality, C=O. In glucose it is positioned on C1 forming an aldose, whilst in fructose it is positioned on C2, meaning that fructose is described as a ketose.
The aldehyde or ketone group can react with a hydroxyl group to form a covalent bond. Formally, the reaction between an aldehyde and the hydroxyl group of a sugar (an alcohol) creates a hemiacetal whereas a ketone reacts with a hydroxyl group to form a hemiketal.
These reactions can also take place intramolecularly so that the straight chain form of the sugar cyclizes. Hence glucose forms a six membered ring structurally similar to that of pyran, whilst fructose forms a five membered ring similar to furan.
Note that a new asymmetric centre is formed on cyclization, at C1. Thus two isomers of D-glucose exist: a-D-glucose (in which the OH group at C1 lies below the plane of the ring) and b-D-glucose (in which the OH group at C1 lies above the plane of the ring). These a and b forms are called anomers and in aqueous solutions they rapidly interconvert via the open chain structure to give an equilibrium mixture. This process is called mutarotation and occurs for a large number of the simple sugars.
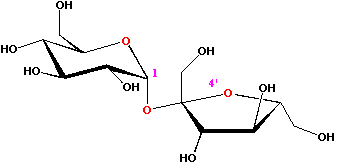
Sucrose, which is composed of fructose and glucose linked together, is a disaccharide; it composes a little over 1% of the composition of honey. Disaccharides form when the aldehyde or ketone group on one anomeric carbon atom of one monosaccharide reacts with the hydroxyl group of a second monosacharide. The covalent bond formed is called a glycosidic bond.