Acids and Bases
from Project
Primary: Chemistry Activities
You have come across acids and bases in everyday life.
Acids are things that taste sour, like vinegar or lemon juice. Bases taste
bitter, like mustard and medicine. This is why cough syrup is always flavoured
– to hide the bitter taste. Our tongue is a sort of detector to sense bitter
or sour tastes and detect whether an acid or a base is present.
Our tongue is not the only acid/base detector. This is good
because not all acids and bases are same to put in our mouths. Other natural
products can be used as well. Red cabbage, red onion and grape juice all turn
different colours in the presence of acidic or basic materials.
These may come
in liquid form or as a strip of coloured paper called litmus paper. When a blue
piece of litmus paper is placed in an acidic solution, the paper turns red. If
red litmus paper is placed in a basic solution, it turns blue.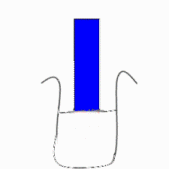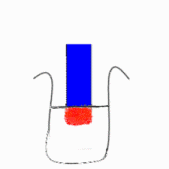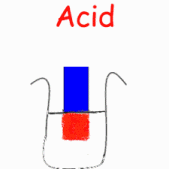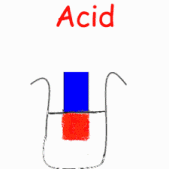
Movie from www.mynewschool.co.uk
The strength of an acid or base,
how bitter or sour it is, is measured on a pH scale which ranges from 1-14. If
the pH is 1-6, the material is sour and acidic; if it is 8-14, the material is
basic and bitter. Seven is neutral pH and is neither an acid nor a base. Most
tap water is very close to a pH of 7. When an acid is mixed with a base, the
chemical properties of the two substances mix and cancel to give a neutral
substance.
You can make your very own pH
indicator using red cabbage. Adult supervision and assistance is necessary.
What you need
-
1/2 small head of red cabbage
-
knife
-
water
-
blender
-
measuring cups
-
measuring spoons
-
strainer
-
re-sealable bags
Your indicator may show a rainbow of colours!
-
hammer
-
clear plastic cups
-
variety of common materials
such as:
-
vinegar
-
clear
ammonia
-
baking soda
-
lemon juice
-
tap water
-
clear soft
drink (7-Up, Sprite)
-
milk
-
salt
-
sugar
-
milk of
magnesia
-
antacid
(Tums, Rolaids)
-
aspirin
-
acetaminophen
(Tylenol)
-
sparkling
mineral water
What to do
To make the indicator:
Chop the cabbage into small pieces, such as 2-cm strips and
place in the blender. Add one part water for every two parts cabbage. Blend for
1-3 minutes until the cabbage is finely chopped. Pour the mixture through a
strainer or sieve to remove the cabbage pieces and keep the liquid. Refrigerate
this until you are ready to use it. The liquid should keep for 2 weeks.
Testing of household products:
Pour a small amount of the liquid products to be tested
straight into the clear cups. The test works best for clear or white substance
because the colour change in the indicator can most easily been seen. For solid
products, such as aspirin or antacids, place the tablet into a re-sealable bag
and use the hammer to crush it into small pieces. Dissolve this in water to make
a testable solution. First add a small amount (approximately a teaspoon) of
indicator to a cup containing only water. What colour does the water and
indicator become? Carefully add a small amount (a teaspoon) of cabbage indicator
to the different samples. Note the colour changes. Which colour indicates an
acidic solution? Which shows a base is present? In
acidic solutions such as vinegar, the indicator turns the solution red. In basic
solutions, such as baking soda, the solution turns bluish after the indicator is
added.
A fun use of cabbage
indicator!
Now that you know how the indicator works,
let’s have some fun with it. For this you will need some soft paper, such as
grey drawing paper, an acidic solution (vinegar water), a basic solution (baking
soda or ammonia water), cotton swabs and a spray bottle with cabbage indicator
inside. On the paper use the cotton swabs to write messages or drawings with the
different solutions. Once the ‘ink’ is dry, spray the sheet with the
indicator to make your messages and drawings ‘magically’ appear in different
colours. Now you can impress your friends with your very own homemade invisible
ink!
Home

