

Mechanism
Friedel Crafts Acylation
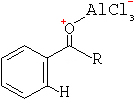
Because of this more than one mole of Lewis acid is needed per mole of benzene for the reaction to reach completion. It is then necessary to hydrolise the complex in H+ solution to liberate the ketone. Carboxylic acid anhydrides are sometimes used instead of acyl chlorides. Acylation involving acid anhydrides is most useful in the case of cyclic compounds.
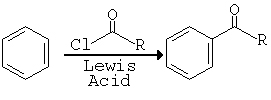
Reaction
Generation of Electrophile
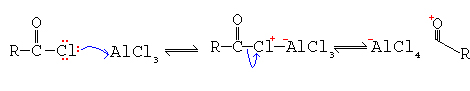
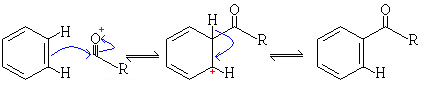
Notes
In a Friedel Crafts Acylation Reaction benzene reacts with acyl halides in the presence of a Lewis acid (an aluminium halide) to give 1-phenolalkanones (phenol ketones). An example of this is the preparation of 1-phenolethanone (acetophenone) from benzene and acetyl chloride, by using aluminium chloride as the Lewis acid. The reaction can be stopped at the monosubstitution stage, the acyl group prevents further substitution because of the electron withdrawing effect of the acyl group. In this reaction there is a formation of a complex between the resulting ketone and the aluminium chloride.
Uses
Monosubstituted alkybenzenes can be synthesised via acylation without the problem of group rearrangement or polysubstitution.