

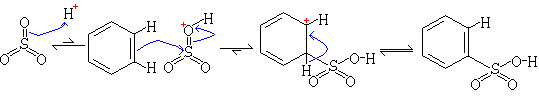
Mechanism
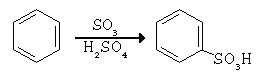
Concentrated sulfuric acid does not sulfonate benzene at room temperature. However a more reactive form called fuming sulfuric acid, permits electropilic attack by SO3. Fuming sulfuric acid is made by adding about 8% of sulfur trioxide, SO3, to the concentrated acid. Because of the strong electron withdrawing effect of the three oxygens, the sulfur in SO3 is electrophilic enough to attack benzene directly. Proton transfer results in the sulfonated product, benzenesulfonic acid.
Reaction
Benzene Web Page: Main | Reactions | Links
Copyright © 2002 Nick Barwell.