
Lavoisier

1743-1794
His Life Story
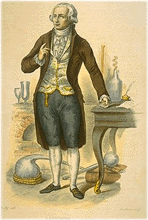 Antoine-Laurent Lavoisier was born in 1743 into a rich bourgeois family in
Paris. His father being a lawyer, he too started off his studies as a law
student. However, this branch never really appealed to him, being far more
attracted by the exciting and mysterious field of science (wise man!).
This interest he pursued passionately and in recognition of his early work, he
was elected into `L'Academie des Sciences in 1768 at the surprisingly young age
of 25. He went on from here to buy into `La Ferme Generale`, the private
corporation of farmers that collected taxes for the crown. This was a very controversial
move at the time. This was in fact the times of peasant unrest in
France that would lead to the revolution and the tax farmers were reputed for
enriching themselves. Lavoisier however always claimed that his only goal was to
raise money for science.
Antoine-Laurent Lavoisier was born in 1743 into a rich bourgeois family in
Paris. His father being a lawyer, he too started off his studies as a law
student. However, this branch never really appealed to him, being far more
attracted by the exciting and mysterious field of science (wise man!).
This interest he pursued passionately and in recognition of his early work, he
was elected into `L'Academie des Sciences in 1768 at the surprisingly young age
of 25. He went on from here to buy into `La Ferme Generale`, the private
corporation of farmers that collected taxes for the crown. This was a very controversial
move at the time. This was in fact the times of peasant unrest in
France that would lead to the revolution and the tax farmers were reputed for
enriching themselves. Lavoisier however always claimed that his only goal was to
raise money for science.
In 1771, Lavoisier married young Marie-Anne Pierrette Paulze who was then but
the tender age of thirteen. This just shows what scientists could get away with
in those days! With time, she was in fact to prove to be a valuable scientific
aide for her husband. She busied herself with learning English so as to
translate the works of Joseph Priestly and others, for Lavoisier, as any self
respecting Frenchman, had never bothered to learn it. She also practiced art and
engraving and so in time illustrated Lavoisier's works and draw sketches of hit
laboratory and experiments.
In 1775, Lavoisier became a commissioner of the royal Gunpowder and Saltpeter administration. Taking up residence in the Paris Arsenal, he there installed his new laboratory that attracted many young chemists from far and wide to learn about his new `chemical revolution`.
As well as a scientist, he was also a social and political activist and as such was involved, as everyone else of his time, in the Revolution. This led to his helping drawing up and contributing to the different reforms in the early post-Revolution years. He is notably known to have contributed to the establishment of the metric system and was a member of its comity. In 1791 he was even appointed Secretary at the Treasury.
Alas, as with many of the great thinkers and revolutionaries of that time, Lavoisier was a victim of the post-Revolution government of Robespierre, popularly known as `La Terreur`. His former connection to `La Ferme Generale` were revealed and he was imprisoned, along with the other 31 surviving former tax farmers in November 1973. Despite his contributions to Science and the services he had rendered to his country, he was found guilty of conspiracy against the people of France and guillotined on 8 May 1794. The mathematician Joseph-Louis Lagrange remarked on this event, "It took them only an instant to cut off that head, and a hundred years may not produce another like it."
His Works
Lavoisier was the architect and father of the `Chemical Revolution` which occurred over the course of the twenty year span from 1770 to 1790. During this period, chemistry underwent such a fundamental reform that nothing like it had been seen before or, some say, since. The notable date here is 1975, date at which he moved to the Paris Arsenal, acquiring new spacious research labs.
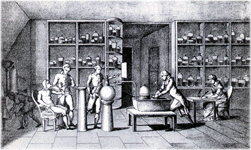
At the heart of Lavoisier's work is his principle that matter -identified by weight- would be conserved through an y chemical reaction. His most important contributions based on this theory was his discovery of a component of air which he named `oxygen` (ring any bells!), and his definitive proof by composition and decomposition that water is composed of hydrogen and oxygen.
His
discovery of oxygen hailed the end of the plogiston theory. This theory was developed
as a result of Boyle's tin calx experiments. These experiments showed
that the tin gained weight as the calx was formed and therefore plogiston was an
imaginary product that was thought to be released on calx formation. This
implied plogiston to have a negative mass, something rather odd overlooked by
most until Lavoisier. He showed through various experiments that metal when
heated would react with a component of air to form a calx but that this
component could later be reformed upon further heating. This component was
oxygen. He went on to conclude that air is composed of two components oxygen and
azote, and that oxygen is essential for combusting, rusting and respiration.
However, this discovery had already been made by Priestly and Lavoisier drew all
credit for his conclusions. Some people say that he had an annoying tendency to
use other people's results without acknowledgement and draw conclusions of his
own.
Lavoisier other major work was the establishment of a chemical nomenclature. He effectively drew up a list of thirty-three substances that he termed elements and gave them new names, some of which are still in use today such as zinc, sulphur, oxygen etc. . However, his list did also include `caloric` and `light` which he thought of as quantifiable substances. He also believed that the existence of atoms was philosophically impossible.
To propagate all of these novel ideas, Lavoisier wrote his book `Traite Elementaire de Chimie`, which was the first real universal chemistry textbook. As well as these discoveries, he is also credited with several smaller works, such as the invention of Plaster of Paris.
Home Laplace Pasteur Marie Curie Du Pont