X-Rays
X-rays can be used in a variety of ways,
depending on the information required. They have been used in connection
with art since 1896.
Visually
 X-rays
can penetrate solid objects in much the same way as they do a human body, seeing
through layers of paint to reveal earlier works, sketches or
repairs. Artists would often use a canvas or wood panel many times
over as they were very expensive. These under paintings as they are called
are present in both fake and real paintings so do not by themselves indicate a
forgery. X-rays of the Mona Lisa reveal that Leonardo painted three
different versions before settling on the one we know today.
X-rays
can penetrate solid objects in much the same way as they do a human body, seeing
through layers of paint to reveal earlier works, sketches or
repairs. Artists would often use a canvas or wood panel many times
over as they were very expensive. These under paintings as they are called
are present in both fake and real paintings so do not by themselves indicate a
forgery. X-rays of the Mona Lisa reveal that Leonardo painted three
different versions before settling on the one we know today.
X-Ray Diffraction
Crystalline solids act as
three-dimensional diffraction gratings for x-rays. This is because the
distances between planes of particles in a crystalline solid are of the same
order as the wavelengths of x-rays. As an x-ray meets a solid it interacts
with electrons and is scattered. This produces a pattern which is distinct
for individual crystal structures. To see the pattern, it must be made
visible; this
is done by using a photographic film. Using this pattern the structure of the solid
is estimated and the diffraction pattern this model would produce is compared
with the experimental one. If the model is not correct it is altered until
a better match is found. Using this iterative method a structure is arrived at.
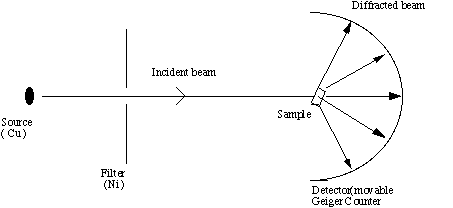
The main use of electron diffraction is
to analyze the components of a pigment which are crystalline.
X-Ray Fluorescence (XRF)
XRF is a technique used to analyze which
elements are present in both a qualitative and a quantitative way. If it
is used for qualitative analysis then it is non-destructive, this is often
essential when analyzing an important piece of work. A qualitative
analysis can often be sufficient to classify a piece of work as either fake or
genuine, if it uses materials unavailable for the time it purports to be from
then it is almost certainly a fake.
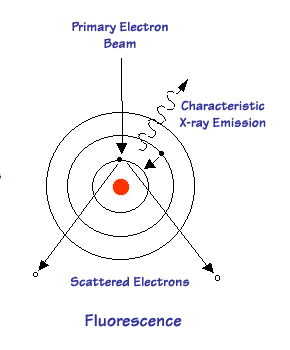 XRF
uses the Photoelectric Effect. A sample is bombarded with x-rays and instead of
the x-rays being scattered as above, they are absorbed. The energy is
transferred to an electron in one of the inner shells, this electron is then
ejected leaving a vacancy. As it is unfavorable for an atom to be in this
high energy state, an electron transfers from an outer shell to the vacancy in
the inner shell returning the atom to its ground state. In this way the
atom emits an x-ray characteristic of a particular element. The energy of
the x-ray corresponds to the binding
energy between the outer and inner shells. The atom is said to fluoresce.
XRF
uses the Photoelectric Effect. A sample is bombarded with x-rays and instead of
the x-rays being scattered as above, they are absorbed. The energy is
transferred to an electron in one of the inner shells, this electron is then
ejected leaving a vacancy. As it is unfavorable for an atom to be in this
high energy state, an electron transfers from an outer shell to the vacancy in
the inner shell returning the atom to its ground state. In this way the
atom emits an x-ray characteristic of a particular element. The energy of
the x-ray corresponds to the binding
energy between the outer and inner shells. The atom is said to fluoresce.
One example where XRF is particularly
useful is ascertaining whether metal objects are modern or ancient. Over
the years methods of production and refining have changed and improved.
Metal made more recently, particularly silver, is much purer than it was in
antiquity. Therefore the presence of many trace elements is a major clue to the
time of origin.
 X-rays
can penetrate solid objects in much the same way as they do a human body, seeing
through layers of paint to reveal earlier works, sketches or
repairs. Artists would often use a canvas or wood panel many times
over as they were very expensive. These under paintings as they are called
are present in both fake and real paintings so do not by themselves indicate a
forgery. X-rays of the Mona Lisa reveal that Leonardo painted three
different versions before settling on the one we know today.
X-rays
can penetrate solid objects in much the same way as they do a human body, seeing
through layers of paint to reveal earlier works, sketches or
repairs. Artists would often use a canvas or wood panel many times
over as they were very expensive. These under paintings as they are called
are present in both fake and real paintings so do not by themselves indicate a
forgery. X-rays of the Mona Lisa reveal that Leonardo painted three
different versions before settling on the one we know today.
 XRF
uses the Photoelectric Effect. A sample is bombarded with x-rays and instead of
the x-rays being scattered as above, they are absorbed. The energy is
transferred to an electron in one of the inner shells, this electron is then
ejected leaving a vacancy. As it is unfavorable for an atom to be in this
high energy state, an electron transfers from an outer shell to the vacancy in
the inner shell returning the atom to its ground state. In this way the
atom emits an x-ray characteristic of a particular element. The energy of
the x-ray corresponds to the binding
energy between the outer and inner shells. The atom is said to fluoresce.
XRF
uses the Photoelectric Effect. A sample is bombarded with x-rays and instead of
the x-rays being scattered as above, they are absorbed. The energy is
transferred to an electron in one of the inner shells, this electron is then
ejected leaving a vacancy. As it is unfavorable for an atom to be in this
high energy state, an electron transfers from an outer shell to the vacancy in
the inner shell returning the atom to its ground state. In this way the
atom emits an x-ray characteristic of a particular element. The energy of
the x-ray corresponds to the binding
energy between the outer and inner shells. The atom is said to fluoresce.