The 2nd law of
thermodynamics
The second law of thermodynamics states:
"Energy spontaneously tends to flow only from being
concentrated in one place to becoming diffuse and spread out."
 This
basically means that you can't get something for nothing, there will always be a
dissipation of energy as heat when producing work. This can be quantified in
terms of a reversible cycle. A reversible cycle has the maximum efficiency of any system
working between Thot and Tcold.
This
basically means that you can't get something for nothing, there will always be a
dissipation of energy as heat when producing work. This can be quantified in
terms of a reversible cycle. A reversible cycle has the maximum efficiency of any system
working between Thot and Tcold.
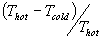
For a simplified Otto cycle the maximum efficiency is given by
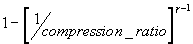
where
gamma is the ratio of heat capacities and the compression ratio is the max : min volume ratio of the engine's cylinder.
(image
taken, without permission from http://www.startribune.com/cars/ )
(reference
'Thermal Physics' R. Baierlein - Cambridge University press 1999)
 This
basically means that you can't get something for nothing, there will always be a
dissipation of energy as heat when producing work. This can be quantified in
terms of a reversible cycle. A reversible cycle has the maximum efficiency of any system
working between Thot and Tcold.
This
basically means that you can't get something for nothing, there will always be a
dissipation of energy as heat when producing work. This can be quantified in
terms of a reversible cycle. A reversible cycle has the maximum efficiency of any system
working between Thot and Tcold.