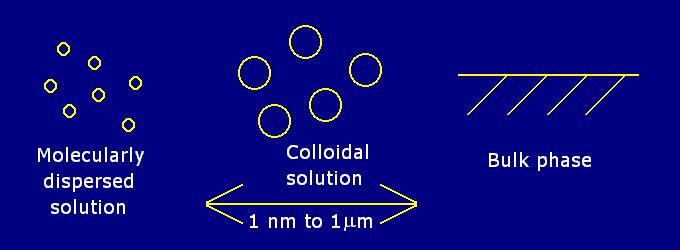
A mixture in which one substance is divided into minute particles (called colloidal particles) and dispersed throughout a second substance. The substances are present as larger particles than those found in solution, but are too small to be seen with a microscope. There are no strict boundaries on the size of colloidal particles, but they tend to vary between 10-9 m to 10-6 m in size.

The mixture is also called a colloidal solution, colloidal system, or colloidal dispersion. The three forms in which all matter exists are solid, liquid or gas. Colloidal systems can be any combination of these states.
A colloidal system is not a true solution but it is not a suspension either because it does not settle out like a suspension will over time.
Colloids are larger than most inorganic molecules and remain suspended indefinitely. They are large molecules, such as proteins, or groups of molecules. They have many properties, depending on their large specific surface.
Colloid formation can be classified in two systems, namely reversible and irreversible. In an irreversible system, the products are so stable or removed so well that the original reactants cannot be reproduced. A reversible system is one in which the products can be made to react to reproduce the original reactants.