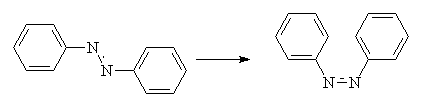
The azo compound class accounts for 60-70% of all dyes. As you might expect, they all contain an azo group, -N=N-, which links two sp2 hybridised carbon atoms. Often, these carbons are part of aromatic systems, but this is not always the case. Most azo dyes contain only one azo group, but some contain two (disazo), three (trisazo) or more2.
In theory, azo dyes can supply a complete rainbow of colours. However, commercially they tend to supply more yellows, oranges and reds than any other colours. Research is always continuing, though, so that now there are some viable blue azo dyes on the market2. The relationship between the colour of an azo dye has been more fully discussed in The Basis of Colour.
Azo dyes give bright, high intensity colours, much more so than the next most common dye class (anthraquinones). They have fair to good fastness properties, but not so good as the carbonyl and phthalocyanine classes. Their biggest advantage it their cost-effectiveness, which is due to the processes involved in manufacture.
The general formula for making an azo dye requires two organic compounds- a coupling component and a diazo component. Since these can be altered considerably, an enormous range of possible dyes are available, especially as the starting molecules are readily available and cheap. Furthermore, the simplicity of the reactions mean that the process can be scaled up or down very easily, which is always a key factor in the cost of chemicals. Energy requirements for the reaction are low, since most of the chemistry occurs at or below room temperature. The environmental impact is reduced by the fact that all reactions are carried out in water, which is easy and cheap to obtain, clean and dispose of. As other dye classes become less viable from either an environmental or economic reasons, azo dyes become ever more attractive options.
As with any double bond, the planar -N=N- bond shows geometrical isomerism:

This change from trans (preferred) to cis can be effected by exposure to UV radiation. This can lead to photochromism, a light-induced reversible colour change in some dyes, for example C.I. Disperse Red 1. This effect was considered a nuisance and has largely been eliminated by careful development of more stable dyes. But photochromic dyes are beginning to make a comeback in technology like sunglasses and sunroofs in cars2.
This involves the removal of a hydrogen from one part of the molecule, and the addition of a hydrogen to a different part of the molecule. This is common when there is an -OH group ortho or para to the azo group:
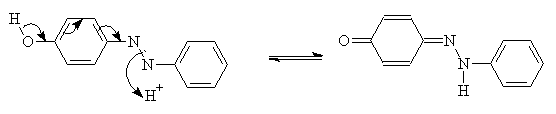
Tautomeric forms can be identified form their characteristic spectra. Ketohydrazones are normally bathochromic compared to their counterpart hydroxyazo forms. Ketohydrazones also have higher molar extinction co-efficients. However, not all azo dyes show tautomerism, and some tautomeric forms are more stable than others2.
An overview of azo dye synthesis is shown below:
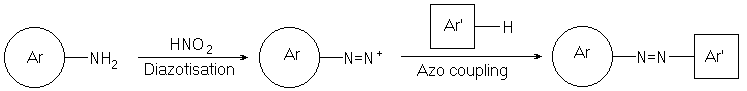
This involves a primary aromatic amine, called the diazo component. It is treated in low temperature, acid conditions with sodium nitrite to form an unstable diazonium salt2.
The diazonium salt is reacted with a coupling component (for example a phenol or an aromatic amine). This forms the stable azo dye.