Cellulose is a high molecular weight polymer composed of b-D-glucose units- it is a polysaccharide. It is produced by higher plants to reinforce their cell walls. Unlike starch, which is composed of a-D-glucose chains, cellulose cannot be digested by mammals. Cellulose is a major component of textile fibres like linen, cotton, paper and viscose2.
Cellulose fibres have -OH functional groups, which can dissociate slightly under the right conditions to give the polymer a negative charge. Cellulose has a fairly open structure which allows larger molecules to move through it. It is best dyed by direct dyes and vat dyes2.
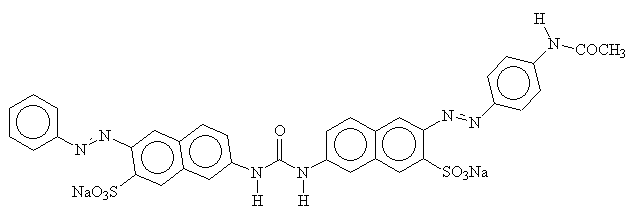
The name 'direct dye' alludes to the fact that these dyes do not require any form of 'fixing'. They are almost always azo dyes, with some similarities to acid dyes. They also have sulphonate functionality, but in this case, it is only to improve solubility, as the negative charges on dye and fibre will repel each other. Their flat shape and their length enable them to lie along-side cellulose fibres and maximise the Van-der-Waals, dipole and hydrogen bonds. Below is a diagram of a typical direct dye. Note that the sulphonate groups are spread evenly along the molecule on the opposite side to the hydrogen bonding -OH groups, to minimise any repulsive effects2.

The main problem with direct dyes is their lack of fastness during washing. However, they are cheap, so are popular for items which are less likely to require fastness during washing (for example paper)2.
Wash fastness may be improved, though, by the application of direct and developed dyes, which contain -NH2 functional groups as well as sulphonate groups. In this process, the dyed fabric is treated with sodium nitrite, which causes the dye to be converted to a diazo salt. It is then treated with a coupling compound such as 2-napthol. The resultant larger azo molecule now has more affinity for the fibres, and is less soluble2.
Vat dyes are a good example of the cross-over between dyes and pigments. Large, planar and often containing multi-ring systems, vat dyes come exclusively from the carbonyl class of dyes (for example, indigo). The ring systems of the vat dyes help to strengthen the Van-der-Waals forces between dye and fibre.
Vat dyes are insoluble in water, but may become solublised by alkali reduction, for example sodium dithionite (a reducing agent) in the presence of sodium hydroxide. For this reason, they tend not to contain many other functional groups which may be vulnerable to oxidation or reduction. The leuco form produced by alkali reduction is absorbed by the cellulose and, once there, can be oxidised back to its insoluble form. Oxidation is usually performed using hydrogen peroxide, but occasionally with atmospheric oxygen under the correct conditions. Treating the dyed textile with a soap completes the process, since the soap molecules encourage the dye molecules to clump together and become crystalline2.
i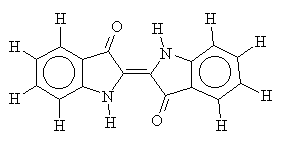
The other types of dyes, for example the azo class, undergo a non-reversible change on reduction.