Everyone is familiar with rainbows- see the top picture for a well known example! Sunlight is refracted by atmospheric water, producing bands of red, orange, yellow, green, blue, indigo and violet. These combined make up white light. If a light source is deficient in any colour band, the light appears to be coloured in the complementary colour. The table below shows wavelength, the corresponding colour, and its complementary colour2.
Wavelength Range/nm |
Colour |
Complementary Colour |
| 400-435 | Violet | Green-yellow |
| 435-480 | Blue | Yellow |
| 480-490 | Blue-blue | Orange |
| 490-500 | Blue-green | Red |
| 500-560 | Green | Purple |
| 560-580 | Yellowy-green | Violet |
| 580-595 | Yellow | Blue |
| 595-605 | Orange | Green-blue |
| 605-750 | Red | Blue-green |
For examples, see below:

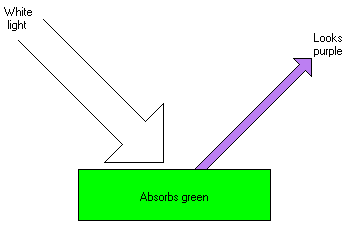
This image shows the effect on white light reflected off a solid object.
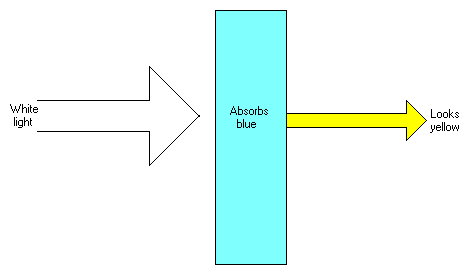
This image shows the effect on white light transmitted through a solution, or other transparent article.
So what exactly causes an object to be coloured?
There are several ways in which molecules can appear to be coloured2
|
Simple molecular excitation, such as in a neon tube, may cause the appearance of colour. This is due to rotation and/or vibration of the molecules. |
|
|
Transition metal complexes are coloured due to the distortion of the metal's d-electron shell caused by ligands surrounding the metal ion. |
|
|
Electronic motion in conjugated organic systems, and charge transfer. |
|
|
Colour in crystalline solids arises from band theory- the blurring of many orbitals through-out the solid. Solids are only coloured if the gap between the Highest Occupied Molecular Orbital (HOMO, the Fermi level) and the Lowest Unoccupied Molecular Orbital (LUMO) is small enough. |
|
|
Colour due to refraction, scattering, dispersion and diffraction- these are all due to the geometrical and physical dimensions of a solid or a solution. |
The first four mechanisms all rely on some form of energy transfer to move either molecules or electrons from their ground state into some excited state. However, only one of these effectively applies to dye molecules, since dye molecules are almost without exception organic conjugated systems. The overlapping p-orbitals effectively mean that no one electron absorbs more energy than another, since all p-electrons in the conjugated system are smeared above and below the molecule. Conjugated organic molecules absorb specific wavelengths of electro-magnetic radiation. If this absorption falls within the visible region, then the light reflected or transmitted is deficient in a particular colour, and the solid (or solution) appears coloured:
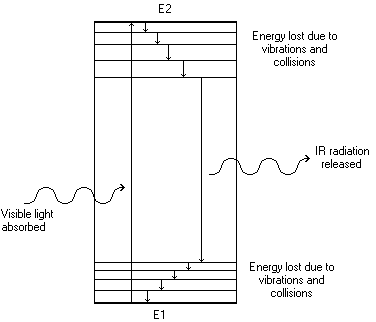
The energy of the electronic transition can be calculated from
DE = hn
where DE is the difference between the two electronic levels, h is Planck's constant and n is the frequency of the absorbed radiation.
The hue of a dye depends on the wavelength it absorbs. Since the wavelengths) the dye absorbs depends on its structure, we can see that any change which affects the p-system will affect the hue. A structural change which causes the absorption band to longer wavelengths (i.e. yellow -> orange -> red -> violet -> blue -> green) is called a bathochromic shift. The reverse shift, towards shorter wavelengths is known as a hypsochromic effect2.
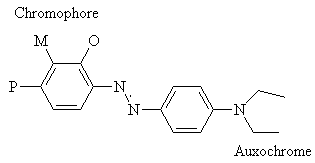
In 1876, Witt proposed that dye molecules contain two groups; the chromophore and the auxochrome. The chromophore is a group of atoms which control the colour of the dye. At that time, Witt suggested that the auxochrome was a salt-forming group, which helped to improve the colour of the dye. His theory was later modified when it was discovered that the chromophore is usually electron-withdrawing, and auxochromes are normally electron-donating. The two groups are connected by a conjugated system2.
So how can a dye molecule's hue be altered? A bathochromic shift may be caused by increasing the electron-withdrawing power of the chromophore (X or Y), increasing the electron-donating power of the auxochrome (Y or X) and by increasing the length of the conjugated system connecting the two2. (It must be remembered that the system shown below is symmetrical, so the chromophore and auxochrome are interchangeable here, but this is not always the case.)
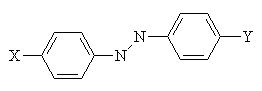
The position of the chromophore and auxochrome also has an effect. In general, the meta position (M) has the shortest wavelength, and the para (P) and ortho (O) absorption wavelengths are approximately equal, and longer than meta. Again this is due to resonance forms.
The molar extinction co-efficient indicates the strength of a dye at low concentrations. It can be calculated using the Beer-Lambert Law
A = ecl
where A is the absorbance of the dye at a particular wavelength, e is the molar extinction co-efficient, c is the concentration of the dye and l is the path-length through the cell2.
The brightness is best described by the shape of the absorbance band; if the band is narrow and sharp, then the dye is bright, if broad, then the dye is dull. This is due to other wavelengths absorbed by the dye, other than the one which causes the hue. The superposition of these determines how broad an absorption band is2.