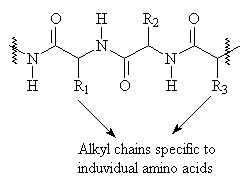
Proteins are composed of long chains of amino acids, which coil up on themselves due to hydrogen bonding and other intramolecular forces. Most protein textiles are derived from structural proteins produced by animals, in particular wool and silk. Wool is largely composed of the same protein as your nails and hair- keratin2.

Proteins contain two major functional groups which affect their ability to interact with dyes: -NH2 which can be protonated to form a positively charged group, and -COOH, which can be deprotonated to from a negatively charged group. They are best dyed by acid dyes and mordant dyes.
Acidic dyes are highly water soluble, and have better light fastness than basic dyes. They contain sulphonic acid groups, which are usually present as sodium sulphonate salts. These increase solubility in water, and give the dye molecules a negative charge. In an acidic solution, the -NH2 functionalities of the fibres are protonated to give a positive charge: -NH3+. This charge interacts with the negative dye charge, allowing the formation of ionic interactions. As well as this, Van-der-Waals bonds, dipolar bonds and hydrogen bonds are formed between dye and fibre2.
As a group, acid dyes can be divided into two sub-groups: acid-leveling or acid-milling2.
These planar dyes tend to be small or medium sized, and show moderate inter-molecular attractions for wool fibres. This means that the dye molecules can move fairly easily through the fibres and achieve an even colour2. This is somewhat similar to the process that occurs during chromatography- the molecules with the strongest affinity for the substrate move the least distance from the point of origin whereas molecules with less affinity move much further. However, the low affinity means that these dyes are not always very resistant to washing2`.
Acid-milling dyes are larger than acid-leveling dyes, and show a much stronger affinity for wool fibres. Because of this, the resultant colour may be less even (see explanation above), but they are much more resistant to washing.
As well as intermolecular interactions, intramolecular interactions play an important part in the properties of the dye. Compare the two molecules shown below. They are isomers, but the one on the right (with hydrogen bonding) shows a much greater resistance to washing in alkali, and much increased light fastness2.
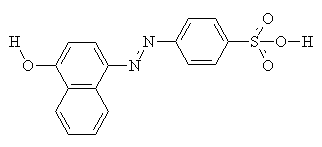
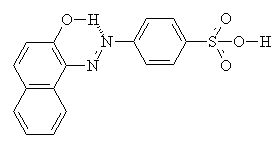
Usually, yellow, orange and red acid dyes are azo compounds, with blues and greens often come from the carbonyl class, particularly anthraquinones (see the example below)2.
An example of an acid dye is Alizarine Pure Blue B. It is a sulphonated aminoanthraquinone, and can be manufactured in two ways1:
|
By condensation of 1-amino-2,4-dibromoanthraquinone with aniline, then treatment under pressure with sodium sulphite in a phenol solvent1. |
|
|
By condensation of sodium 1-amino-4-bromoanthraquinone-2-sulphonate (bromamine acid) with aniline and a copper salt1. |
Mordant is a Latin word meaning 'to bite'13. Mordants act as 'fixing agents' to improve the colour fastness of some acid dyes, which have the ability to form complexes with metal ions. Mordants are usually metal salts; alum [KAl(SO4)2.H2O] was commonly used for ancient dyes, but there is a large range of other metallic salt mordants available. Each one gives a different colour with any particular dye, by forming an insoluble complex with the dye molecules. Chromium salts such as sodium or potassium dichromate are commonly used now for synthetic mordant dyes. The diagrams below show C.I. Mordant Black 1 with and without a chromium (III) ion. Chromium (III) forms 6-coordinate complexes, so two Mordant Black molecules would attach to one ion. Only one is shown below for clarity.
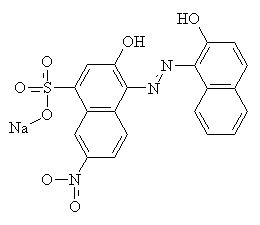
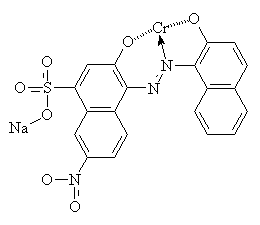
Mordants do not have to be metal salts. Organic molecules such as tannic acid and tartaric acid can be used as well.