These are man-made polymers, made up of monomers with specific characteristics. The polymers most commonly used for clothing are polyesters, polyamide (nylons), and acrylics2.
Polyesters, and cellulose acetate (a semi-synthetic polymer) are dyed with disperse dyes. Polyamides have functional groups similar to proteins, so may be dyed with acid dyes or with disperse dyes. For the dyeing of acrylic fibres, basic dyes are primarily used2.
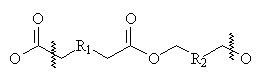
The general structure of a polyester is shown below:

They tend to be fairly hydrophobic (though this depends on the structure of R1 and R2), but not as much as, say, a long hydrocarbon would be, since the -COOC- groups cause some polarity. The polymer chains in a sample of polyester are highly crystalline (for a polymer) and quite tightly packed together. The result of this is that polyesters have very little affinity for large ionic dyes- the dyes simply cannot either distribute between the chains, or form satisfactory intermolecular interactions. Therefore, acid and direct dye classes are useless for this polymer2.
Disperse dyes have low solubility in water, but they can interact with the polyester chains by forming dispersed particles. Their main use is the dyeing of polyesters, and they find minor use dyeing cellulose acetates and polyamides. The general structure of disperse dyes is small, planar and non-ionic, with attached polar functional groups like -NO2 and -CN. The shape makes it easier for the dye to slide between the tightly-packed polymer chains, and the polar groups improve the water solubility, improve the dipolar bonding between dye and polymer and affect the colour of the dye. However, their small size means that disperse dyes are quite volatile, and tend to sublime out of the polymer at sufficiently high temperatures2.
The dye is generally applied under pressure, at temperatures of about 130oC. At this temperature, thermal agitation causes the polymer's structure to become looser and less crystalline, opening gaps for the dye molecules to enter. The interactions between dye and polymer are thought to be Van-der-Waals and dipole forces2.
The volatility of the dye can cause loss of colour density, and staining of other materials at high temperatures. This can be counteracted by using larger molecules, or making the dye more polar (or both). This has a drawback, however, in that this new larger, more polar molecule will need more extreme forcing conditions to dye the polymer2.
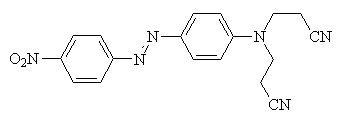
The most important class is the azo class. This class of azo disperse dyes may be further sub-divided into four groups, the most numerous of which is the aminoazobenzene class. This class of dye can be altered as mentioned before, to produce bathochromic shifts. A range of heterocyclic aminoazobenzene dyes are also available. These give bright dyes, and are bathochromically shifted to give blues. The third class of disperse dye is based on heterocyclic coupling components, which produce bright yellow dyes. The fourth class are disazo dyes. These tend to be quite simple in structure. Other than these, there are disperse dyes of the carbonyl class, and a few from the nitro and polymethine classes. Below is an example of a disperse dye2. It is the same as the chime molecule at the top of the page.
Basic dyes possess cationic functional groups such as -NR3+ or =NR2+. The name 'basic dye' refers to when these dyes were still used to dye wool in an alkaline bath. Protein in basic conditions develops a negative charge as the -COOH groups are deprotonated to give -COO-. Basic dyes perform poorly on natural fibres, but work very well on acrylics2.
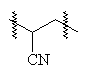
A general structure of an acrylic type polymer is shown below. It is simplified, and doesn't show any anionic groups which are often present2.
The most common anionic group attached to acrylic polymers is the sulphonate group, -SO3-, closely followed by the carboxylate group, -CO2-. These are either introduced as a result of co-polymerisation, or as the residues of anionic polymerisation inhibitors. It is this anionic property which makes acrylics suitable for dyeing with cationic dyes, since there will be a strong ionic interaction between dye and polymer (in effect, the opposite of the acid dye-protein fibre interaction). An example of a basic dye is shown below2:
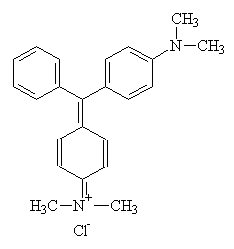
In this case, the charge is delocalised across the molecule, but in some basic dyes, the charge is located on a single nitrogen atom2.
The structure of nylon 6,6 and nylon 6 are shown below:
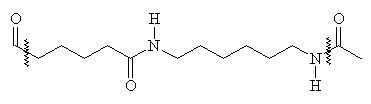
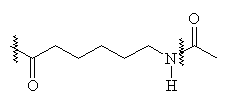
It can be seen that there is a distinct similarity between these polymers and that of keratin (the nylons lack R groups, and have more space between each amide group). The dyeing of these polymers is quite similar to the acid dye action on wool2.