The Basics of Diving Chemistry
Differences between Air and Water
| SEA WATER | AIR | FRESH WATER | |
| Description | Liquid with dissolved minerals | Mixture of gases | Liquid |
| Composition | Water Molecules + Dissolved Minerals | 21%Oxygen + 78%Nitrogen+ 1% Other Gases | Water Molecules |
| Weight per Cubic Foot (lbs) | 64 | 1/12 | 62.4 |
| Maximal Weight per Volume | All is same at all depths | Depends on Pressure | Is same at Depths |
| Compressible | No | Yes | No |
| Freezing Pt | 24oF | -500oF | 32oF |
BOYLE'S LAW
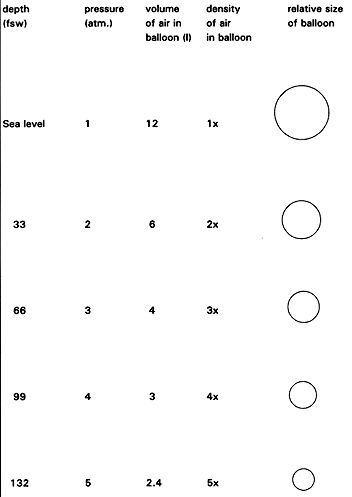
Boyle's Law predicts changes in pressure with depth.
e.g. A balloon filled with 12 litres of air at the surface is taken under water. As the pressure increases the volume inside the balloon decreases hence the balloon shrinks(Only shrinks because it is compressible). When the balloon is returned to the surface, the balloon expands back to its original volume.

Change in balloon pressure(atm), volume(liters) and Relative Density of Air, from Sea Level to 132 Feet (fsw= feet sea water)
Click Here to see How Boyle's Law Affects Divers?
ARCHIMEDE'S PRINCIPLE
This principle determines the buoyancy of a diver in water.
This famous scientist observed that an object immersed in water sinks or floats depending on the weight of water it displaces. If the weight of water displaced is less than the object's weight, it sinks; if the displaced water weighs more, the object floats; and if the displaced water is the same the object is neutrally buoyant.
It is important that a diver manages to establish the correct buoyancy whilst in the water. An experienced diver should be able to reach a point where he/she can be neutrally buoyant by just breathing. The sensation of diving is similar to that of being in space. On the surface of under water, gravity still has an effect, but it can be nullified by establishing neutral or positive buoyancy.

DECOMPRESSION
The deeper the body goes the more bubbles of gas go into the body tissues. This in itself is not a problem, it is the ascent that create problems for the body. During decompression, gases are seeking for pathways to get out of the body tissue.
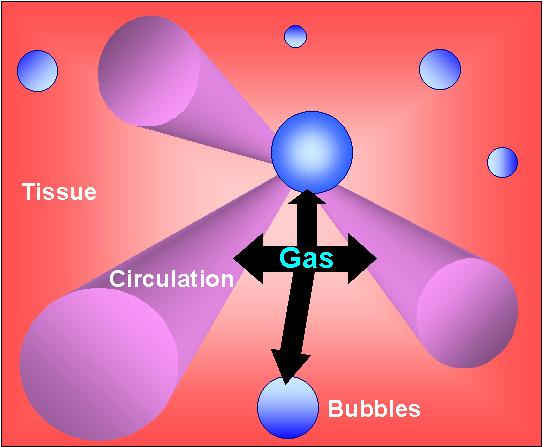
To avoid problems such as "The Bends", it is important that the tissue gas remains in solution while ascending so it can be eliminated by diffusion to the circulation. Therefore, to avoid the painful possibility of going through the bends, if there is any doubt that there are still bubbles in the diver; then he/she should remain deep thus remaining pressurised.
In order to understand decompression and hence decompression sickness, the chemistry of bubbles needs to be looked at more closely
CLICK BELOW: