BUBBLE CHEMISTRY
An easy understanding of how bubbles work can be gained by looking at a glass of fizzy drink.
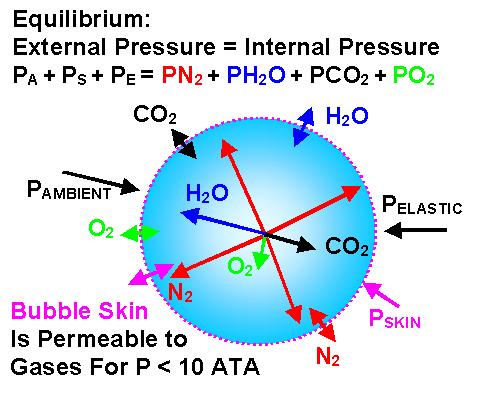
The pressure inside the bubble is generally different from that outside the bubble. The size of a bubble is closely linked to its internal pressure through effects such as skin tension, the elasticity of surrounding tissue and Boyle's Law. The key to keeping the size of the bubble minimal is to keep the internal pressure of the bubble greater than tissue tension. In order to achieve this the correct mixture of breathing gases needs to be chosen.
A bubble remains stable in size if pressure inside = pressure outside

There are several conditions under which bubbles grow or shrink. These conditions can be studied by considering what factors affect changes in bubble size.
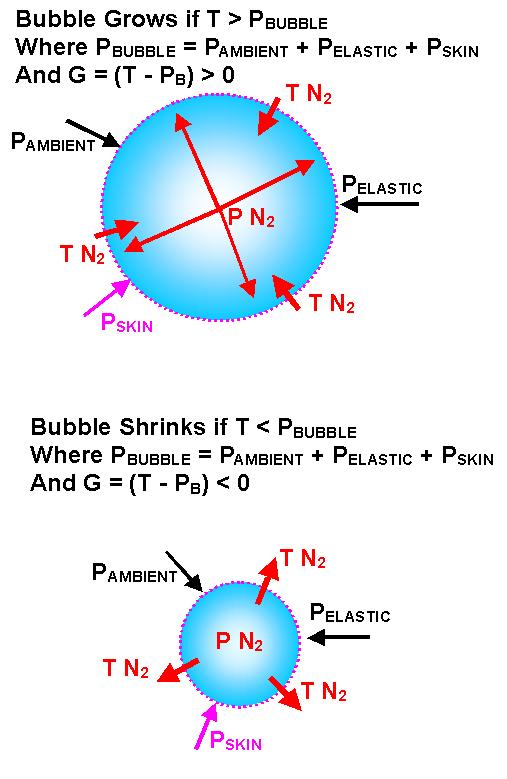
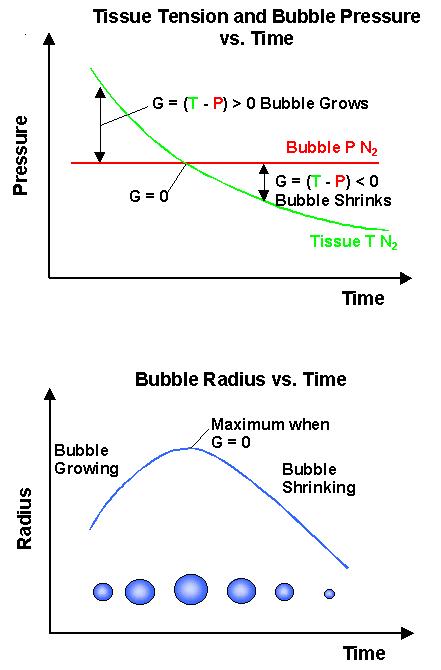
A diffusion equation describes how each gas flows between tissue and bubble. This equation is distinct from the simple rate equations. The lower of the next graphs shows how the radius of a bubble will change according to the difference between its internal pressure and the tension of gas dissolved in the surrounding tissue.
We must not forget however, the source of our original problem: The dissolution of inert gases in the body. Optimal inert gas elimination depends on how much the gas is dissolved in tissues or free in bubbles. The following graphs shows how nitrogen can be eliminated according to two different strategies.
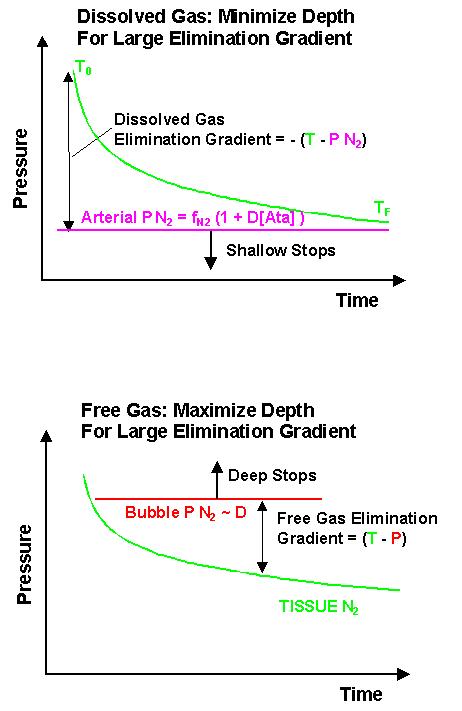
The dissolved gas elimination is maximised by reducing the arterial tension to teh lowest possible value. This is accomplished by either ascending to reduce the depth D or by reducing the Nitrogen fraction (fN2) of the breathing mixture.