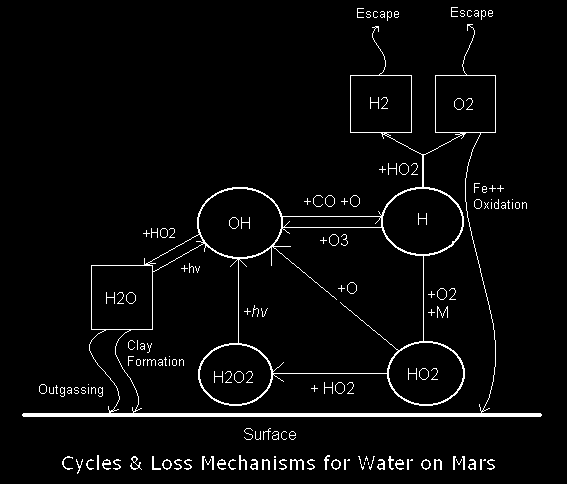
The first constraint
The first constraint, the probable significant excess of oxygen over carbon dioxide, suggests the importance of reactions other than
CO2 + hv ® CO + O
As sources of oxygen. Carbon dioxide photolysis is energetically possible out to a wavelength of 2270 Å, at which the photon energy (hv) is exactly equal to the CO-O bond strength. But real discussion processes put some energy into modes other than bond breaking, such as vibrational excitation of the CO fragment or translational kinetic energy of both fragments; thus in reality photons with wavelengths longer than about 1700 Å have no practical opportunity to dissociate CO2.
Dissociation of CO2 is followed by: O + O ® O2 as the main source of oxygen, because this sequence provides a molar O2 : CO ration of 1:2, whereas observations suggest a number about four times higher. This two-body reaction is very slow because of the very difficult requirement that the energy of bond formation be dissipated over the duration of a single O-O collision. Such reactions can be greatly accelerated in the presence of a third collision partner, which can help dissipate the released energy through translational, vibrational, rotational, and even electronic excitation:
O + O + M ® O2 + M
Reconstruction of carbon dioxide via: CO + O ® CO2 is also terribly slow because the bond energy released during O addition is very large; disposing of this energy during a simple two-body CO-O collision is impractical. Thus here also a three-body process is favored: CO + O + M ® CO2 + M
Some O must always be rendered unavailable for CO + O reactions by forming oxygen, so some CO (in stoichiometric proportions of one CO molecule per O atom or of two CO molecules per O2 molecule) must be produced. Thus, because of the basic reaction stoichiometry, even if this mechanism were very effective, it would still not explain the observed excess of molecular oxygen over CO.

Dissociation of water is the only other plausible source of oxygen. In the presence of water,
H2O + hv ® OH + H
O + H + H
O + H2
Of which the first is by far the most important. Solar UV can drive this reaction photons with wavelengths out to about 1900 Å. Hydroxyl radical can react with H atoms to reconstitute water via the three-body reaction
OH + H + M ® H2O+ M
Which is again faster than the corresponding two-body reaction. Oxygen atoms can react with molecular oxygen via: O + O2 ® O3 to make ozone or with hydroxyl radical via
O + OH ® HO2
To make hydroperoxyl radical, which reacts with H or O:
H + HO2 ® H2O2
H + HO2 ® OH + OH
O + HO2 ® OH + O2
OH can help oxidize CO oxidation by: OH + CO ® H + CO2
Which is much faster than the reaction between CO and HO2,
HO2 + CO ® OH + CO2
Both O2 and O3 are vulnerable to UV photolysis,
O2 + hv ® O + O
O3 + hv ® O2 + O
With O2 photolysis effective out to a wavelength of about 1800 Å. Ozone is energetically very vulnerable, because even photons of 1 m m wavelength have enough energy to remove an O atom; in practice, however, ozone is destroyed by photon in the 2000- to 4000- Å wavelength range, long enough so that shielding by the much more abundant H2O and CO2 is impossible. The solar flux is large and increasing very rapidly with wavelength in this region.