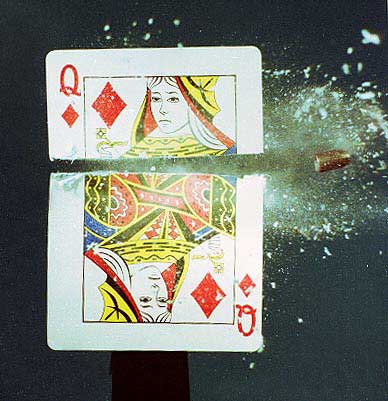
FIREARMS
Gunshot
Residues:
On firing a gun, the explosives that propel the bullet cause a cloud of smoke to erupt from the barrel, leaving a residue of chemicals on the hands of the person holding the gun. Analysis of these residues can give us information about the type of gun that was fired and roughly when it was fired.
Well-known
explosives used in ammunition and the making of terrorist bombs:
Glycerol + nitric acid = nitroglycerin (“NITRO”)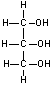
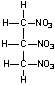
T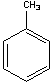 oluene
+ nitric acid = trinitrotoluene (“TNT”)
oluene
+ nitric acid = trinitrotoluene (“TNT”)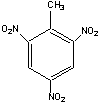
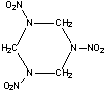 Cyclotrimethylenetrinitramine
(RDX)
Cyclotrimethylenetrinitramine
(RDX)
All of the above compounds are rich in carbon, nitrogen and hydrogen and release large volumes of gas at high pressure when they are burned. As these gases build-up, the pressure inside the gun increases rapidly, forcing the bullet out. The resulting residue is a mixture of by-products from the combustion of the detonator, the initial propellant and metal vapours from the bullet itself.
Generally, the bullet is slightly larger than the barrel of the gun. As the bullet is propelled from the gun, grooves on the inside of the barrel cut into the bullet. This is what gives bullets their spin and stabilises their flight. Amazingly, a bullet can travel at more than twice the speed of sound and spin at a rate of 4000 revolutions per minute. As a result, it is quite possible to be shot and killed by a bullet, without even hearing the gun go off!
One way of analysing the chemicals deposited by gunfire is to make a mould of the hand using paraffin wax to form a thin ‘glove’. This wax can then be examined by bombarding it with neutrons. This can detect the presence of metals such as mercury, lead, antimony and barium, all of which are common components of gunshot residue.
Sticky tape can also be used to remove tiny specks of residue and these can then be analysed using back scattered electron imaging (BEI) and secondary electron imaging (SEI).
One very important method that is also used in many other fields of forensic science is known as atomic absorption (AA). The basis of this is that different elements absorb and emit light at different wavelengths; for example, sodium absorbs and emits light with a wavelength of 589 nanometres. When a sample of the element is held over a flame, light of this characteristic wavelength, and therefore colour, is emitted, which can often be used to identify that particular element. In order to analyse a mixture of elements, such as those found in gunshot residues, a solution of the mixture is vaporised by a flame, through which is shone light thought to be characteristic of an element in the mixture. If there is any of this element present, it will absorb the light, leaving dark lines in the absorption spectrum.