 (ref.7)
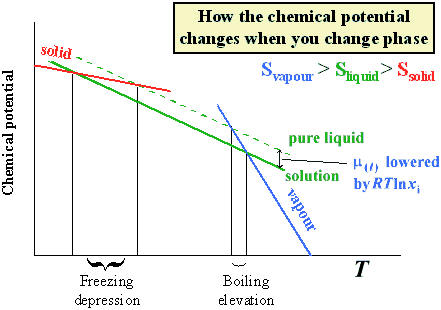
(ref.7)A solution generally has a boiling point which is elevated over that of the pure liquid, and a freezing point which is lower. This is the basis for putting salt (or grit) on the icy roads in winter, to lower the freezing point enough that the ice finds itself above its new freezing point and returns to the liquid state. The elevation of the boiling point and depression of the freezing point are known as colligative properties.
The reason for these colligative properties is that the chemical potential of the solvent has been lowered. This can be seen from the following diagram.
 (ref.7)
(ref.7)
The freezing point depression is given by:
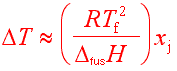
Where x = mole fraction
As you can see, the melting point depend only on the number of solute molecules.
In ice cream the solute that depresses the freezing point is sugar (sucrose).
 (Ref.8)
(Ref.8)
The physical basis behind this is entropy. At the normal freezing point molecules will lose entropy by becoming a solid. But when a solute is present there is extra randomness in the solution. This increases the tendency of the solid to break up. So the solid can melt more easily, i.e at a lower temperature than before.
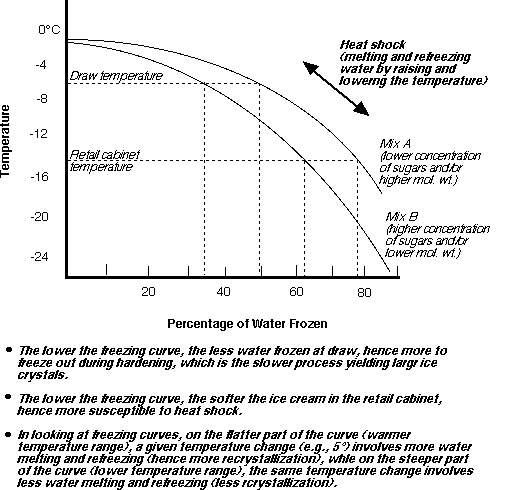
In ice cream as the water freezes the amount of liquid water avaliable for the sucrose to dissolve in decreases such that the sugar solution becomes more concentrated. As a result the freezing point of the remaining solution depresses even further. As more ice forms, so the solution becomes more concentrated and its freezing point continues to drop such that nearl 20% of the water is still liquid at -25°C
The graph below shows the freezing depression of water in ice cream.
 (ref.9)
(ref.9)