|
||||||||||||||||||||||||||||||||
World of Colour
|
The physical measurement of colour | |
Measuring Concentrations using absorbance, | |
Transition Metal Solutions, |
Rhodopsin and the eye
Basics behind Dyes and Pigments
Phosphorescence and Fluorescence
A Thermochromic example
Colour Therapy
Detection using Optical Sensors
Symbols, Glossary, Disclaimer, Credits, References, feedback..
Action 3: Book turning. This image is taken from gifs (ref. 14) and is copyright restricted according to the source given (i.e. it is not the authors' own work).
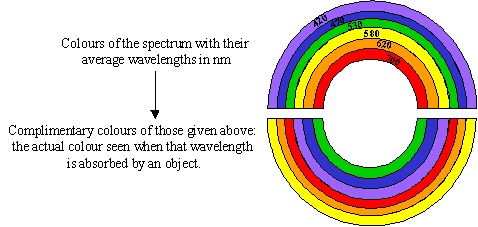
Figure 9: The Colour Rainbow
Pigments date back as far as Neanderthal Man (found in burial sites) and the wall-paintings of the Stone Age (around 15 000BC): yellow and red oxides, soot, black manganese oxide and white porcelain clay made up the natural range at the time. After another 10, 000 years the Egyptians developed some more startling coloured pigments including ultramarine blue; three “terre verte” greens from glauconite, malachite and chrysocolla; orpiment lemon-yellow; and realgar bright red. The synthetic pigments of the time included white lead, red lead, lead antimoniate (yellow) and Egyptian blue. The Greek and Roman civilizations only appear to have developed a bright red cinnabar on top of those already known. The development of blue pigments across the centuries follows a series of mistakes and mishaps within the mineral-extraction community: Firstly, the lack of permanence and expense of extraction led to a limited use of ultramarine blue and therefore an equivalent was desperately needed. In 1704 Diesbach, a German paint manufacturer, mistakenly discovered potassium ferri-ferrocyanide (Prussian blue). When a synthetic ultramarine was made in 1787, the permanence of Prussian blue (along with ultramarine) was still being improved. After indantrone was discovered in 1901, the most commonly used modern day blue pigment Monastral Blue B, basically phthalocyanine with copper (and not iron), was first synthesized in 1928. Today pigments are mainly used in printing inks, car finishes and wall paints.
Bronze Age man extracted
natural dyes from bright flowers in order to colour textile fibres but most of
those known to be used at the time (e.g. Saffron) would not have withstood much
sunlight or a decent wash! The pre-treatment with alum followed by alkali (known as
‘mordanting’) results in improved fastness and was used initially with the
red dye madder. Some classic natural dyes and their origin:
|
Colour |
Name |
Origin |
Comment |
|
Blue |
Indigo |
Isatis tinctoria – European woad Indigofera tinctoria of India |
Precursor indoxyl forms indigo if oxidised in air. |
|
Scarlet |
Kermes |
Coccus ilicis – insect of the Middle East |
For wool and silk |
|
Red |
Madder |
Rubia tinctorium |
For vegetable fibres only |
|
Purple |
Tyrian |
Molluscs of the Mediterranean coasts secret yellow liquid |
Fabric turns from yellow, through green and blue to purple. |
|
Varies |
Haematin |
Logwood of Mexico |
|
If iron – dark blue shades
If chromium – dark blue but black when topped with yellow dyes.
Synthetic organic dyes span a wide range of varying chemical structures including among others azo-, quinones, carbonyls, nitros and phthalocyanines. An independent investigation into the structure, chemistry and applications of all these groups would be needed in order to justify them, but here is where I refer the reader to one of many comprehensive books on dyes and pigments which has been the source of the above information and provides an in-depth up-to-date study of most dye classes: Christie (ref. 4).
Colour
mixing: Additive mixing of coloured light is when the source
is observed directly by the eye. The additive primaries are red,
green and blue
and a common situation of such is in a colour TV where the three primary
phosphors transmit all colours to the eye. Subtractive colour mixing is when the
colours are observed after transmission or reflection by an object. The
subtractive primaries are the exact opposite to additive, i.e. magenta,
yellow
and cyan and a classic situation for these is in printing inks.
