|
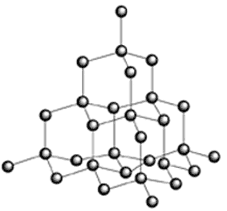
Diamond The allotropes of carbon include diamond, graphite and buckminsterfullerene. Diamond is the most hard, most dense and the least reactive of all the allotropes, it also has no colour. Its properties can be explained by the arrangement of bonds and atoms (figure 1).
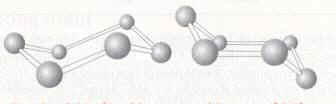
Figure 1. Diamond There are two types of diamond, the most rare has atoms which are very close to one another in the form of a hexagon, and the classic diamond, which has a cubic close packed structure, thus the atoms are also close to one another. Each atom has a coordination number of four so the atoms are arranged in a tetrahedral structure. The carbon atoms are linked with single bonds. The difference between the two polymorphs is the structure of the carbon. Carbon, when it exists in the form of a cyclic six membered ring can change its geometry according to the energy of the molecule and the other atoms around it. It can hold the position of a chair or a boat (figure 2).
The chair is the most stable because the position had the least energy, all the atoms in cubic diamond have this structure. Sometimes, however the conformation can change and some hexagons take the form of the boat, thus the structure of diamond changes. Diamond is an insulator because of the separation of energy (greater than three electron volts) between the highest of filled bands and lowest of unfilled bands in the band theory diagram. Diamond is used for the tips of drills, other industrial appliances where such strength is required, and of course jewellery.
Home ||
Diamond ||
Graphite ||
Buckyballs ||
Nanotubes ||
Fullerenes
Samantha Shanley, School of Chemistry, University of Bristol | ||||