
Types of Snake Venom
As explained in the introduction venomous snakes can be classified into three classes the snake venoms for two are explain below:
1) The elapines, short front fangs (Proteroglyphs) snakes, which include the cobra, mamba, and coral snakes, their venom is neurotoxic (nerve toxins) and paralyses the respiratory centre. Animals that survive these bites seldom have any sequelae (aftereffects of the snake bite such as tissue damage).
2) The two families of viperines, the true vipers (e.g., puff adder, Russell's viper, and common European adder the only venomous snake in the UK) and the pit vipers (e.g., rattlesnakes, copperhead, and fer-de-lance). Viperine snakes have long, hinged, hollow fangs (Solenoglyph); they strike, inject venom (a voluntary action), and withdraw. Many bites by vipers reportedly do not result in injection of substantial quantities of venom. Viperine venom is typically haemotoxic (blood toxins), necrotising (death of tissue), and anticoagulant (preventing the blood from clotting), although a neurotoxic component is present in the venom of some species, e.g., the Mojave rattlesnake
There are approximately 20 types of toxic enzymes found in snake poisons
throughout the world known to man. Although no venomous snake has all of these
toxins, most snakes employ between six to twelve of these enzymes in their venom.
Each of these enzymes has its own special function. Some aid in the digestive
process, while others specialize in paralysing the prey. This is a list of
various toxins that have been identified in snake venom: Proteolytic enzymes
Phosphomonoesterase
Arginine ester hydrolase
Phosphodiesterase
Thrombin-like enzyme
Acetylcholinesterase
Collagenase
RNase
Hyaluronidase
DNase
Phospholipase A2 (A)
5'-Nucleotidase
Phospholipase B
L-Amino acid oxidase
Phospholipase C
Lactate dehydrogenase
Adenosine triphosphatase
Enzymes
Enzymes are biological (virtually always proteins) catalysts. A catalyst is a compound that increases the rate of the reaction but does not affect the yield or equilibrium of the reaction. The enzymes in the snake venom can speed up chemical reaction going on in an organism so much, that they can kill the organism. Enzymes bind temporarily to one or more of the reactants of the reaction they catalyse. In doing so, they lower the amount of activation energy needed and thus speed up the reaction
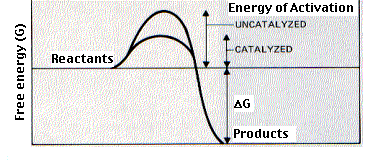
The function of some of the key enzymes have been summarised in the list below and described in greater detail down the page.
Cholinesterase : attacks the nervous system, relaxing muscles to the
point where the victim has very little control.
Amino acid oxidase : plays a part in digestion and the triggering of
other enzymes, (is responsible for venom's characteristic light yellowish
colouring.)
Adenosine triphosphatase : believed to be one of the central agents
resulting in the shock of the victim and immobilizing smaller prey. (probably
present in most snakes.)
Peptide bradykinin potentiators :- Greatly enhance one of the body’s
natural responses to injury (dilation and increased permeabilty of blood
vessels, stimulation of pain receptors, and contraction of some smooth
muscles), thereby enhancing diffusion of venom in the bloodstream, increasing
bleeding, and stopping the ability to flee. Bothrops, Rattlesnakes contain this
venom.
Polypeptide toxins : Directly disrupt nerve-impulse transmission,
usually causing heart or respiratory failure Taxon with the compound in its
venom: Mambas and colubrids : Cobra (Naja)(cobratoxin), Cobra (Naja)
(cardiotoxin), Mojave Rattlesnake (Crotalus scutulatus) (Mojavetoxin), Vipera
(viperptoxin).
Proteolytic enzymes : Catalyse the breakdown of structural components of
tissues. All venomous species contain this venom.
Hyluronidases : Catalyse reactions that break mucopolysaccharide links
in connective tissues, thereby enhancing diffusion of venom. Several types of
snake contain this venom.
Proteases : Catalyse reactions that disrupt protein peptide bonds in
tissues, causing blood-vessel wall damage and haemorrhaging and muscle-fibre
deterioration. Vipers, pit vipers contain this venom.
Phospholipases : Catalyses reactions that harm musculature and nerves.
Almost all venomous species (e. g., phospholipase A, in Copperheads,
cottonmouths, Fer-de-lances, Rattlesnakes, Cobras, Vipera)
Thrombin like enzymes : Inhibit blood clotting. Vipers, pit vipers, a
few elapids (but rare) contain this venom.
Nerve growth factor (an enzyme) : Stimulates the growth of nerve cells.
Copperheads and Cottonmouths, Rattlesnakes contain this venom.
Action : Disrupt normal cellular function, causing death of the affected
cells. Vipers and elapids (occurrences vary) contain this venom.
Glycoproteins : Suppress normal immune response of tissues through
anticomplementary reactions. Some vipers contain this venom.
Biogenic amines : Disrupt normal transmission of nerve impulses and
other types of signalling between cells. Cottonmouths, Copperheads,
Rattlesnakes, Tree vipers, contain this venom.
Other enzymes : ribonucleases, deoxyribonucleases, nucleotidases,
lactate dehydrogenases, acidic and basic phosphatases.
Cholinesterase (neurotoxins)
Cholinesterase is one of many important enzymes needed for the proper functioning of the nervous systems of humans and other animals. A synapse is an electrical switching centre in the body's nervous system, in which a muscle is being directed by a nerve to move. An electrical signal, or nerve impulse, is conducted by acetylcholine across the junction between the nerve and the muscle (the synapse) making the muscle to move. Normally, after the muscle has moved, acetylcholinesterase is released which breaks down the acetylcholine terminating the stimulation of the muscle. The enzyme cholinesterase accomplishes this by chemically breaking the acetylcholine into other compounds and removing them from the nerve junction. If cholinesterase is unable to breakdown or remove acetylcholine, the muscle will continue to move uncontrollably.
Electrical impulses can fire away continuously unless the number of messages being sent through the synapse is limited by the action of cholinesterases. Repeated and unchecked firing of electrical signals can cause uncontrolled, rapid twitching of some muscles, paralysed breathing, convulsions, and in extreme cases, death.
Unlike the acetylcholine molecule; however, snake venom molecule will not immediately react with the alcohol group of the receptor site, and therefore, it will not break down and vacate the receptor site. Instead it will permanently interact with the synapse causing the channels of the receptor site to remain open, causing a draining of the electrical impulses. When the impulse is drained, the muscle fibre does not receive sufficient stimulation. Since the muscle response is a collective effect created by multiple muscle fibres, only third of the receptor sites in the diaphragm need to be blocked for muscle function to stop occurring. If this occurs, the victim usually dies within 30 minutes. The only way to save the life of a victim of snakebite with high levels of neurotoxins such as that of a cobra is to inject the appropriate antivenin shortly after the patient has been bitten or put him on an artificial respirator.
Phospholipase A2 (PLA2)(neurotoxin)
Phospholipase A2 (PLA2) like Cholinesterase is a common neurotoxin present in snake venom. Various neurotoxins have been found to have similar amino acid sequences. Inhibitors of various venom proteases are important to understand their biological role and ways that their enzymatic activity can be altered.
PLA2 disrupts biological membranes and can lead to permanent damage or even lysis (splitting or breaking of cells). The body secretes its own versions of PLA2 (pancreatic [I] or non-pancreatic [II]) that have totally different functions. Human PLA2 aid in: digestive enzymes, cell contraction, cell proliferation, destruction of pathogens (Disease producing organisms) Venom PLA2 is classified as group III and has a similar structure to I & II only when bound to a receptor. The various physiological effects of PLA2 are determined by the type of receptor to which it binds. Receptors include N- receptors (neurological- III) and M-receptors (muscular- bind only I & II). It may act pre- or post-synaptically at the neuromuscular junction by binding to acetylcholine receptors (N-receptor). The binding of PLA2 to acetylcholine receptors block the binding of acetylcholine, which causes flaccid (limp) paralysis. The binding of the receptor affects in a variety of ways in different muscles. This suggests that there are differences in affinity of the binding in different muscle types. Respiratory failure often accompanies the paralysis because there is likely a high affinity for PLA2 in phrenic nerve-diaphragm endplate receptors.
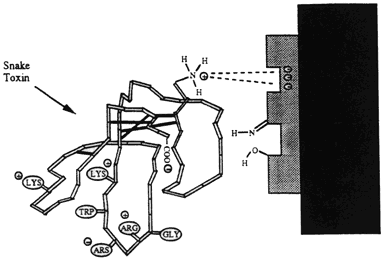
Image taken from http://www.engin.umich.edu
L-amino acid oxidase (LAO)
L-amino acid oxidase (LAO) catalyses the oxidative deamination of a number of L-amino acids:
![]()
Absolute decomposition for L-isomers amino acids of :-
leucine, isoleucine, norleucine, a-amino butyric acid, phenylalanine, tyrosine,
tryptophan norvaline, methionine, histidine and citrulline. L-serine,
threonine, aspartic acid, glutaric acid, lysine and ornithine are only slightly
attacked. 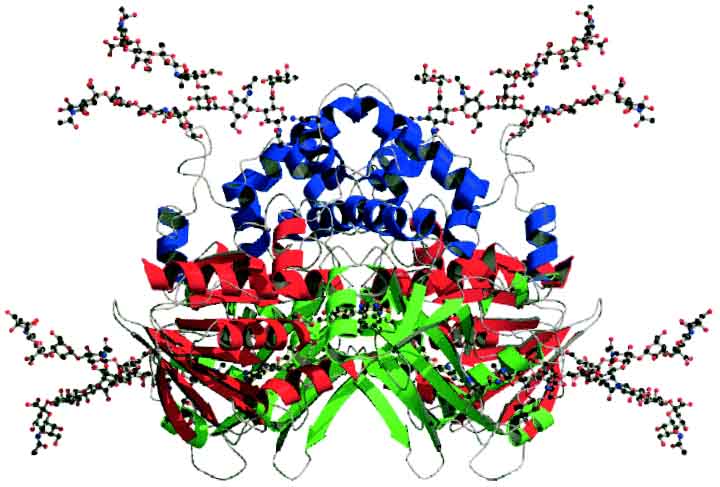
X Ray crystalography of Malayan pit viper Calloselasma rhodostoma L-amino-acid oxidase.
image taken from http://www.blackwell-synergy.com
Hyaluronidase
Hyaluronidase is involved in the inflammatory response of venom, with the softening of tissue and the facilitation of flow of the other substances. It causes the following reaction to occur to hyaluronic acid in the body:
Hyaluronic acid -------------> Acetylglucosamine
Hyaluronic acid (HA) is everywhere in humans, with the highest concentration found in soft connective tissue. It plays an important role for both mechanical and transport purposes; e.g. it gives elasticity to the joints and rigidity to the vertebrate disks, and it is also a constituent of major importance in the vitreous body of the eye. It is a linear polysaccharide (long-chain biological polymer) formed by repeating disaccharide units with glucosamine links. HA is distinguished from the other glycosaminoglycans, as it is free from covalent links to protein and sulphuric groups. This glycosaminoglycan has been demonstrated to be important to tissue functions such as tissue hydration, lubrication, solute transport, cell migration, cell function and differentiation. It is a polymer made up of monomer units as shown below:
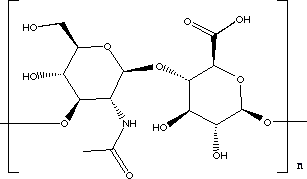
Monomer of hyaluronidase image taken from http://www.chem.ox.ac.uk/mom/hyaluronidase/hyaluronidase.html
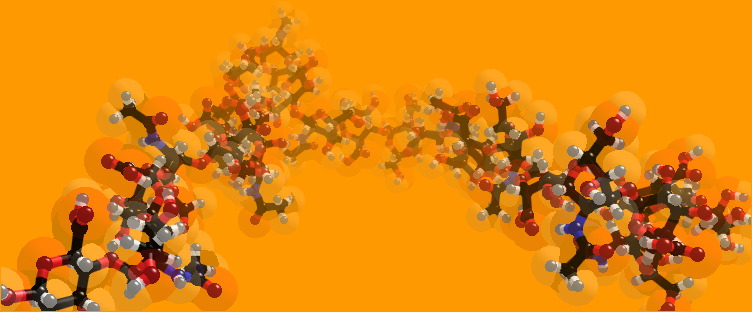
polymer of hyaluronidase image taken from
http://www.chem.ox.ac.uk/mom/hyaluronidase/hyaluronidase.html
Proteases
Catalyse reactions that disrupt protein peptide bonds in tissues, causing blood-vessel wall damage and haemorrhaging and muscle-fibre deterioration
Metalloproteases
(Vipers, pit vipers)
A metalloprotease is a protease (hydrolyses proteins) that uses a metal ion as a catalyst. Snake venom often contains many metallo-proteases, some of which are hemorrhagic (profuse bleeding) toxins. Metalloproteases often contribute to severe necrotic changes in tissues after envenomation. Venom metalloproteases have physiological effects like tissue invasion, haemorrhage, necrosis (death of cell), and perhaps apoptosis. Metalloproteases can also be caseinolytic (Casein is protein in milk that is used for a variety of functions.) When the isolated metalloprotease was placed with various muscle types, fibrinogen muscle was hydrolysed (splitting into fragments by the addition of water) and a clot was formed. Metalloproteases are calcium-dependent and slightly inhibited by ATP and ADP (likely because they compete for the metal ion.)
Fibrinogenolytic Proteases
Venom that is shown to be toxic has many proteases that have a variety of physiological effects. Those that are fibrinogenolytic have haemorrhagic activities.