Terpenes
The terpenes form the largest group of natural odorants, and thus also the largest group of modern fragrance ingredients. Thousands of different terpene structures occur in perfume ingredients, both natural and synthetic. Table 1 shows the odour type and approximate annual consumption of some of the highest tonnage terpene fragrance ingredients.
| Material | Odour | Approximate usage (tonnes/annum) |
| Amberlyn | Ambergris | 6 |
| Carvone | Spearmint | 600 |
| Citronellol and esters | Rose | 6000 |
| Dihydromyrcenol | Citrus, Floral | 2000 |
| Geraniol-nerol and esters | Rose | 6000 |
| Hydroxycitronellal | Muguet | 1000 |
| Borneol/isoborneol and acetate | Pine | 2000 |
| Linalool | Floral, wood | 4000 |
| Linalyl actate | Fruit, floral | 3000 |
| Menthol | Mint, coolant | 5000 |
| (Methyl) ionones | Violet | 2000 |
| Terpineol and acetate | Pine | 3000 |
| Acetylated cedarwood | Cedar | 500 |
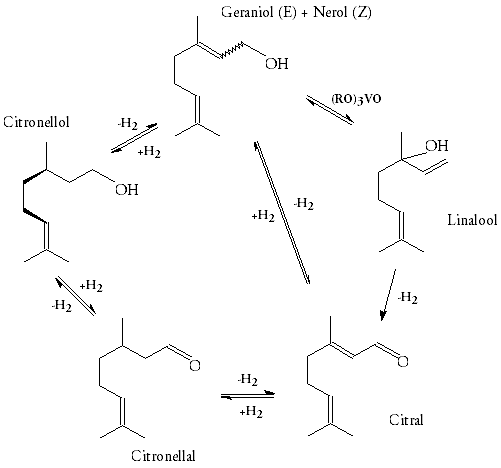
Geraniol-nerol, linalool, citronellol, citronellal and citral are five of the most important terpenes for the perfume industry. Their alcohols and esters are also important. They are also key starting materials for other terpenes. The scheme of reactions below shows the structures of these materials and how they can be interconverted by isomerisation, hydrogenation and oxidation.

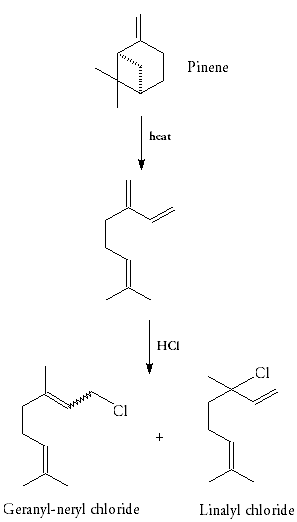
Their sources are mainly from turpentine or petrochemical sources. Water-insoluble crude sulfate turpentine is obtained from the conversion of softwood (pine, fir, spruce) into paper in the Kraft process. Fractional distillation of this crude sulfate turpentine gives a number of products, such as pinenes, dipentenes,etc. The ratio of the products depends on the type of tree used. These crude products are then converted to one of the 5 main terpenes many possible reactions. One of the earliest reactions, the pyrolysis of pinene is shown in Scheme 2 below.