 These have the same molecular
mass, but chemical properties such as susceptibility to hydrolysis and
chromatographic behaviour are structure dependent. Ellagitannins are
produced by the oxidative coupling of galloyl groups in gallotannins.
These have the same molecular
mass, but chemical properties such as susceptibility to hydrolysis and
chromatographic behaviour are structure dependent. Ellagitannins are
produced by the oxidative coupling of galloyl groups in gallotannins.
Structure
Tannins are subdivided into two groups:
1) Protoanthocyanidines (also called condensed, because of their structure)
2) Hydrolysable tannins (gallotannins, ellagitannins)
Protoanthocyanidines are polymeric structures containing two to fifty flavanoid units, a diverse group of metabolites based on heterocyclic ring systems derived from phenylalanine and polyketide biosynthesis. Their name is derived from the reaction with acid butanol. This acid-catalysed oxidation yields an important class of polyphenolic structures called the and of the blue colours found in fruit. They are also responsible for the astringent taste of wine and some fruit juices. The most widely studied protoanthocyanidines are epicatechin and catechin. Their antioxidant, anti-hepatitis and their potential anti-cancer properties have attracted the attention of many medical researchers. On the left, a dimer of catechin-epicatechin is shown.
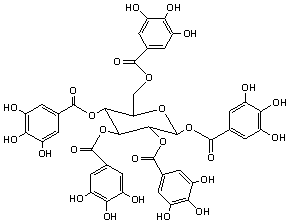
Gallotannins are formed from the reaction of glucose with dimers or higher
oligomers of gallic acid (Figure 3 below). The beta anomer is the most common in
nature. Due to their complex structure, gallotannins have many isomers.
 These have the same molecular
mass, but chemical properties such as susceptibility to hydrolysis and
chromatographic behaviour are structure dependent. Ellagitannins are
produced by the oxidative coupling of galloyl groups in gallotannins.
These have the same molecular
mass, but chemical properties such as susceptibility to hydrolysis and
chromatographic behaviour are structure dependent. Ellagitannins are
produced by the oxidative coupling of galloyl groups in gallotannins.
(Figure 3)
Home Occurrence Structure Reactivity