Until
recently, cubane was considered as just an academic curiosity,
a novelty with no actual use within modern day chemistry.
Yet further research found that serveral possible applications
in industry, materials science and medicine could exist, giving
cubane a new future of which Philip E. Eaton had once visualised:
"There
is something for these compounds that are being made, and
they do, I think, have a very interesting future - both in
the explosives area and potentially in the pharmaceutical
area." - Philip E. Eaton
The main areas
of interest are:
Explosives and Propellants
Material Science
Pharmaceuticals
Explosives
and Propellants
There
are several factors which dictate how good an explosive a
compound would make. Major factors are the number of moles
and molecular weights of the gaseous products and the energetics
of decompostion. Density is also an important factor as the
more moles of an explosive that can be packed into a smaller
volume the better due to the fact that the explosion pressure
is proportional to the density of the material, squared.
Cubane
is amongst the dozen densest saturated hydrocarbon molecules
that exist, with a density of 1.29
g cm-3. It is thermodynamically unstable with a
heat of formation of 144
kcal mol-1. These two factors alone led the US
Army Armament Reseacrh and Development Centre (ARDEC) towards
a proposal for a brand new, powerful explosive - Octanitrocubane,
(ONC) where all of the hydrogens are replaced by nitro groups,
bound to each carbon through nitrogen:
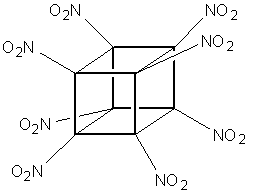
Octanitrocubane
C8(NO2)8
Octanitrocubane
contains enough oxygen to oxidise all carbon atoms to CO2
and, along with dinitrogen, explodes into 12 gaseous molecules
producing the desired effect for either explosives or propellants:
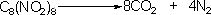
Predictions
are that ONC has the potential to become one of the most powerful
explosives, the estimates for some of it's properties are
shown here against other military standards:
Like
cubane, ONC was once thought impossible to make and keep however
when finally synthesised, it was found to be fairly stable
with decomposition taking place at over 220 ºC and it
was very dense. It was fairly shock resistant, meaning that
it has the potential to become a useful explosive. The only
problem is that cubane derivatives are still far too expensive
to be considered as the starting material of an explosive
that may be required in thousand-pound quantities for defense
projects or major demolition work, limiting it's use for the
time being.
In
terms of propellants, cubane derivatives such as ONC fit the
specification perfectly. Releasing the explosive burst of
enegy required, yet with no hydrogen atoms present, no water
is produced during the burning and so produce little or no
visible smoke (steam) in the plume behind the rocket; such
'low-signature' rockets are very hard to track.
Return
to the top of the page
Material
Science
Cubanes
have a very defined geometry, rigid and of regular shape and
dimensions. This could allow them to act as building blocks
in the developing world of nanoarchitecture, in the form of
oligomeric compounds.Polycubanes, alkylated [n]cubylcubanes,
can already be synthesised:
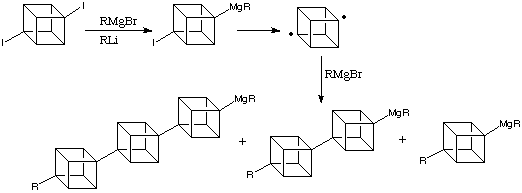
A
possible application of this would be to synthesise liquid
crystals with new, exceptional properties such as UV transparency.
Return
to the top of the page
Pharmaceuticals
Cubanes
are not inherently toxic. They are considered as biologically
stable, lipophilic platforms on which a wide choice of substituents
in a variety of well defined spatial relationships can be
installed.
The
distance across the cube (diagonally) is very similar to that
of the ipso-para distance across a benzene ring; meaning that
functional groups can be not only added within the 'benzene-plane'
but above and below it. This creates a huge opening for research
in the field of substituted cubanes as replacements for bezyl
groups within drug development as they would offer similar
behaviour yet more options and maybe new solutions as they
would provide more sites for substitution.
It
has already been discovered that dipivaloylcubane -a cubane
derivatized with keto, cyano, and amide groups (shown below)
exhibits moderate activity against human immunodeficiency
virus (HIV) without causing any damage to healthy cells within
the body.
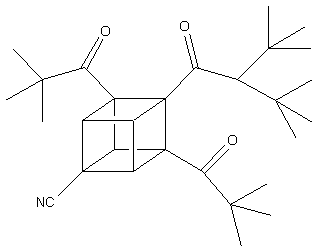
Also,
a phenylcubane derivative has been known to show some anti-cancer
properties just showing the versatility and maybe the pharmaceutical
basis for further applications of cubane and its derivatives.
|