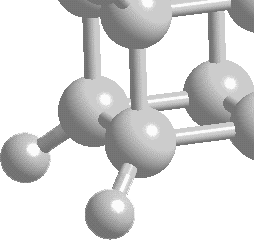
| Cubane
(C8H8) is named appropriately; its skeleton is in the shape
of a cube. At each corner of this cube there is a carbon atom
(carrying a hydrogen) bound to three identical neighboring
carbons. This molecule was thought only to exist in theory
up until it was synthesised in1964 by Philip E. Eaton, a professor
at the University of Chicago, and ever since has it proved
fascinating, providing some unique chemistry.
Taken
from www.wellesley.edu/Chemistry/Flick/molecules/cubane.html
Can't
See Anything?
It
was such a radical molecule at the time, and still is, as
the whole system is highly strained. The internuclear C-C-C
bond angles sit at 90º, far from the tetrahedral angle
of 109.5º. This causes huge amounts of energy to be stored
within the bonds of cubane and so gives birth to a whole range
of applications.
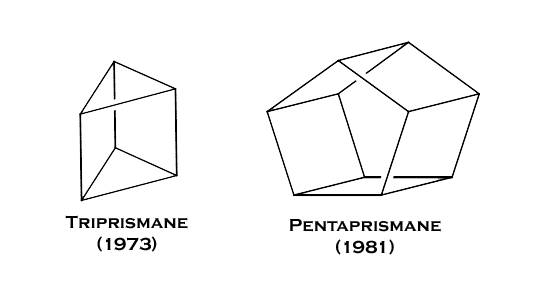
Cubane
was the first from a growing family of highly strained ring
sytems which have been synthesised in the years following
cubane. Triprismane and pentaprismane are two other such examples:
|