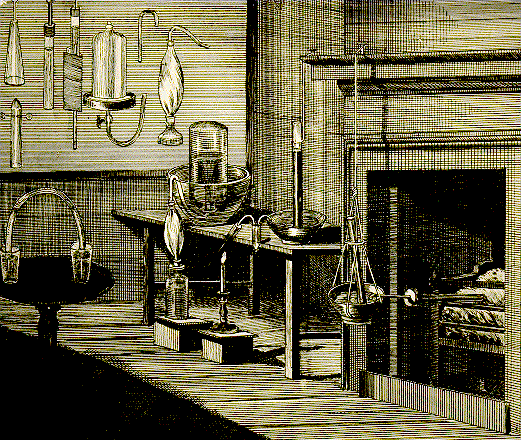
Priestleys laboratory
Priestley: The Scienist and the Discovery of Oxygen

Priestleys laboratory
Despite much work on gases and electricity Priestley will still be remebered most for his role in the discovery of oxygen, but as so often happens we must take one step back to go one step forward.
The Theory of Phlogiston (1669)
It is believed that matter is made from the elements air, water,
and 3 elements of earth terra pinguis (fatty earth), mercurial,
and fusible. On combustion it was thought that the fatty,
combustible earth burnt away during combustion. In 1703 George
Stahl renamed the terra pinguis as phlogiston, to take into
account the corrosion of metals to be the "matter and
principle of fire, not the fire itself". Contained in
all combustible matters escaping from all burning material. For
example a piece of wood turning to ashes on burning, the burning
is explained by the escape of the phlogiston from the wood
originally composed of ashes and phlogiston.
Carl Scheele
 Swedish chemist, a laboratory assistant in Uppsala
working with little money and simple apparatus. Around 1773 he
discoverd what he called fire air (oxygen) due to ability of
things to burn vigorously in oxygen. Firstly fire air was made by
mixing nitric acid with potassium hydroxide to form potassium
nitrate. Then distilling with sulphuric acid to form NO2;
with was removed by absorption through calcium hydroxide, and
oxygen. Then also forming oxygen, by heating calx of mercury
(mercury oxide) to form mercury and oxygen.
Swedish chemist, a laboratory assistant in Uppsala
working with little money and simple apparatus. Around 1773 he
discoverd what he called fire air (oxygen) due to ability of
things to burn vigorously in oxygen. Firstly fire air was made by
mixing nitric acid with potassium hydroxide to form potassium
nitrate. Then distilling with sulphuric acid to form NO2;
with was removed by absorption through calcium hydroxide, and
oxygen. Then also forming oxygen, by heating calx of mercury
(mercury oxide) to form mercury and oxygen.
However, due to the relative isolation and lack of money in sweden the wider scientific community, sadly did not hear of his discovery until 1777, when his book "Chemical Treatise on Air and Fire" was published, 3 years after Joseph Priestley published his results.
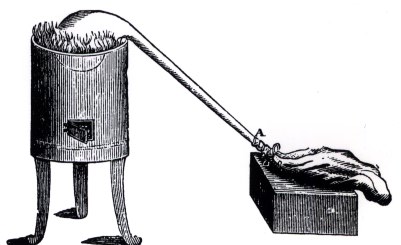
Scheele's Appartus for extracting Oxygen
Jospeh Priestley
 Working independently for Scheele, and in a very
similar method heating mercury oxide to produce oxygen and
mercury. In his own words:
Working independently for Scheele, and in a very
similar method heating mercury oxide to produce oxygen and
mercury. In his own words:
"1st of August 1774, I endeavored to extarct air from mercurius calcinatus [mercury oxide]; and I presently found that, by means of this lens, air was expelled from it very readily. ... I admitted water to it [the extracted air], and found that is was not imbibed by it. But what surprized me more than I can well express, was, that a candle burned in this air with a remarkably vigourous flame ... I was utterly at a loss how to account for it." Experiments and Observations on Different Kinds of Air, 1775
The lens spoken of by Priestley, was a burning glass used to focus the suns rays and heat up the sample, in the same way that a magnifying glass can be used to set fire to a piece of paper. Preistley went on to show that a mouse lived twice as long when enclosed in pure oxygen than in normal air, and finding that air contains about one fifth oxygen.
But what was the new air discovered by Priestley and Scheele. Priestley working from phlogiston theory, an the fact that a candle when burning gives out phlogiston, and assuming that the candle goes out when the air is saturated with phlogiston. Assuming therefore that the new air allows the candle to burn more vigorously there must be little or no phlogiston in the air. Thus he called the new air 'dephlogisticated air'. Despite having all the right results his belief in the phlogiston theory lead him astray, so now enter:
Antoine Lavoisier
 Despite claims that he had played a part in the
discovery of oxygen, this is not true as he had heard of both
Scheele's and Priestley's work before he started his work on
oxygen. What set aside Lavoisier's work was the systematically
quantitive methods in his research, taking inspriation from
others notably Boyle and Black (discoverer of latent heat and
carbon dioxide) who also advocated the use of quantitive methods.
Despite claims that he had played a part in the
discovery of oxygen, this is not true as he had heard of both
Scheele's and Priestley's work before he started his work on
oxygen. What set aside Lavoisier's work was the systematically
quantitive methods in his research, taking inspriation from
others notably Boyle and Black (discoverer of latent heat and
carbon dioxide) who also advocated the use of quantitive methods.
"The whole art of making experiments in chemistry is founded on the principle: we must always suppose an exact equality or equation between the principles of the body examined and those of the products of its analysis."A Lavoisier, Traite Elementaire de Chimie, 1789
Lavoiser found that on burning sulphur and phosphorus an increase in weight occured when burnt in air. By burning phosphorus in a bell jar above mercury he noted that some of the gas had been absorped due to an increase in the height of mercury in the jar. In the gas the remained after combustion a lit taper extinguished, indicating that no oxygen was present after combustion.. The remaining powder (P2O5) was far heavier than the mass of the phosphorous burnt. With about 1g of phosphorous forming 2.7g of powder. Through futher experimentation he produced a new theory of combustion in which the respirable air (oxygen) combines with the chemical combusting. Completley the opposite to the phlogiston theory. Despite resistance from notably Watt and Priestley, Lavoisier showed his theory was correct and giving the new air the name oxygene. Lavoisier was the first to appreciate the discovery of oxygen, and that it was an element in itself, paving the way for Dalton's atomic theory, combined with a quantive approach Lavoisier started the move towards modern chemistry that we have today.
Scheele, Priestley and Lavoisier, all knew of the importance of oxygen to life, Lavoisier continued to carry out research into respiration, and this now leads us full circle back to the demonstration in the lunar room. Now though armed with the knowledge of evolution, the steam engine, how to find the temperature in our kilns, and perhaps the most fundamentally that it is the lack of oxygen, or as the lunar men would have called it dephlogisticated air, that would cause the bird to die, not as previously believed the lack of the element air itself.
Sadly it is now time to return to the 21st Century, but next time your on a train spare a though for the men of the Lunar Society who made it all possible.
Lavoisier image from; http://www.homeoint.org/morrell/articles/pm_origin.htm,
lab image from http://www.english.upenn.edu/~jlynch/Frank/Gifs/prlab1.html
Scheele image from: http://www.college.ru/chemistry/course/content/chapterr/section1/paragraph114
Bibliography