
Is it Safe?
Studies have shown that 75-80% of ingested sodium benzoate leaves the body within 6 hours, and all is gone by 10 hours. The natural metabolic process in the body occurs in the liver and combines sodium benzoate with glycine to produce hippuric acid, which can then be excreted. This will remove around 95% of all sodium benzoate in the body; any remaining can be detoxified with glycuronic acid.
The problem with sodium benzoate is the benzene ring. Benzene has been listed as a highly poisonous chemical which can increase the likelihood of cancer, in particular leukemia.

Taken from: http://www.le.ac.uk/eg/safety/coshh/phrases.htm
Benzene can be formed from sodium benzoate by the addition of ascorbic acid (vitamin c) and these two are often found in conjunction with each other as ascorbic acid is also added to drinks as an antioxidant and to prolong shelf life.
The mechanism involves the ascorbic acid reacting with metal atoms such as iron or copper dissolved in the drink to create hydroxyl radicals. As we know, under acidic conditions found in the carbonated drinks sodium benzoate breaks down into benzoic acid. The hydroxyl radical attacks the benzoic acid, evolving carbon dioxide and leaving just benzene in the drink. This problem is now controlled in the US by the addition of the chelating agent calcium disodium EDTA.
The food and Drug Administration (FDA) has labeled sodium benzoate as safe, but however it has imposed a limit (in the US and Europe) to the amount allowed in drinks. This limit currently stands at 1 part par billion, the same as the standard for drinking water.
So the answer to "is it safe?" is yes, subject to the limits imposed by law.
-
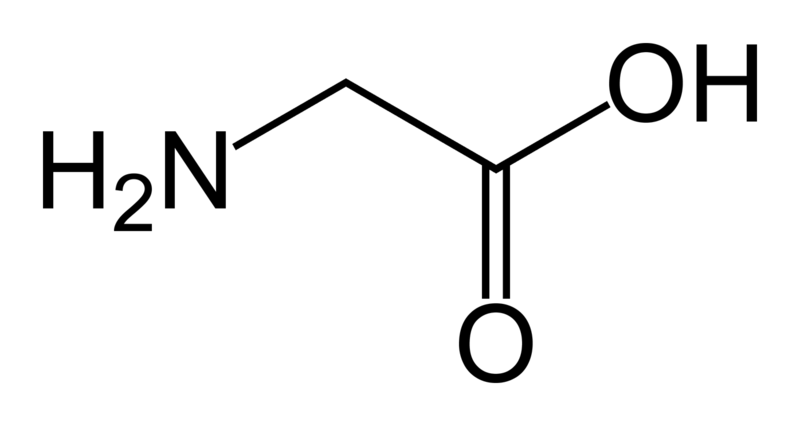 This is is the skeletal formula of
glycine.
This is is the skeletal formula of
glycine.
Taken from http://en.wikipedia.org/wiki/Glycine
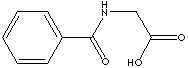 This
is the skeletal formula of hippuric acid.
This
is the skeletal formula of hippuric acid.
Taken from http://www.chemicalland21.com/lifescience/pha
- Glycuronic acid is the uronic acid of glycose, which has the skeletal formula s follows.
![]()
Taken from: http://www.chem.qmul.ac.uk/iupac/2carb/glo.html
-
Radicals are species with an unpaired electron, they are highly reactive and usually "steal" an electron from the nearest stable species.