 (taken
from
www.chm.bris.ac.uk/ motm/aspartame/aspartameh.html)
(taken
from
www.chm.bris.ac.uk/ motm/aspartame/aspartameh.html)Properties and use
Aspartame as mentioned in the introduction has a very sweet taste and minimal bitterness. Its appeal as a sweetener comes from the fact that it is approximately 180 times sweeter than the sugar sucrose but has a far lower calorific value. This is because as it is around 180 times more sweet than sugar, the amount of aspartame that is required to achieve a given sweetness level will be less than 1% of the amount of sugar required meaning over 99% of the calories can be replaced. This makes it very useful in foods and drinks in this day and age where many people are trying to cut down on their calorie intake but still want the same sweet taste.
Aspartame is most commonly used in diet soft drinks which have a very similar sweet taste to the sugar versions of the drinks but of course contain a fraction of the calories.
 (taken
from
www.chm.bris.ac.uk/ motm/aspartame/aspartameh.html)
(taken
from
www.chm.bris.ac.uk/ motm/aspartame/aspartameh.html)
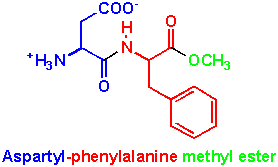
Aspartame has a structure as shown above. As can be seen it is composed of aspartic acid, phenylalanine and methanol. When it is metabolized by the body it is broken back down to these three components.
1. Aspartic acid is an amino acid found commonly in nature which is naturally produced in the body. As with many compounds in the body its level is carefully regulated. Any excess is broken down and does no harm to the body.
2. Phenylalanine is also an amino acid but unlike aspartic acid it is not produced in the body so must be obtained through diet.
3. Methanol is an alcohol commonly found in many foods. Although poisonous in large quantities, small volumes are easily dealt with by the liver which metabolizes it so it can be excreted.