Synthesis
As with many organic compounds, when synthesizing aspartame, stereoisomerism has to be considered. Because of their tetrahedral shape and bonding arrangements, these compounds can exist as non-superimposable mirror image forms. These are called enantiomers.
Phenylalanine and aspartic acid which are two components of aspartame have two isomers which are non-superimposable mirror images. They are described as being chiral. If the incorrect isomers of these components are used in the synthesis of aspartame then the molecule will not have the correct shape to fit with the binding site of the receptors on the tongue which detects sweetness.
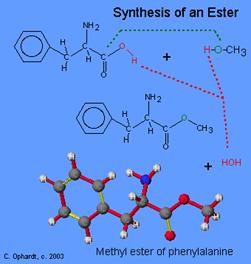
Therefore when the synthesis is carried out equal quantities of the two compounds are reacted using the desired isomers. The first stage in the synthesis is the reaction of phenylalanine with methanol. This is an ester reaction and is carried out in the presence of hydrochloric acid.
This gives a phenylalanine methyl ester

(taken from http://www.elmhurst.edu/~chm/vchembook/549aspartame.html)
The final stage is to react this methyl ester of phenyalanine with the aspartic acid. This reaction is complex and it is worth noting that the acid group on the aspartic acid has to protected so that it does not react.
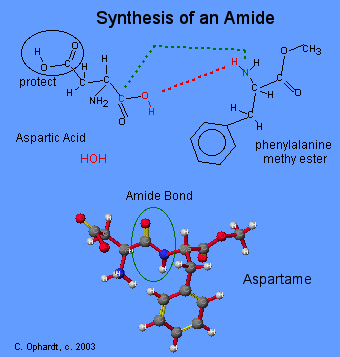
(taken from http://www.elmhurst.edu/~chm/vchembook/549aspartame.html)